Story At a Glance
-
History of Leading Edge Clinic’s Cancer Program– researching and developing complementary cancer care protocols
-
Overview of Drug Repurposing: Exploring the concept of using existing non-cancer drugs for oncology, highlighting its potential to address unmet needs in cancer treatment.
-
The ReDO Project: Introducing the Repurposing Drugs in Oncology (ReDO) Project, its mission, and its focus on identifying and testing well-characterized drugs for cancer treatment.
-
Leading Edge Clinic’s Role: Detailing the clinic’s involvement in research, including the Rebuild Medicine study on repurposed drugs for cancer.
-
Case Studies and Evidence: Highlighting real-world examples and emerging scientific evidence supporting the efficacy of repurposed drugs in oncology.
Introduction
At Leading Edge Clinic, we specialize not only in treating post-COVID and post-COVID-19 vaccine syndromes but also in integrative, repurposed drug treatments for cancer. Our approach utilizes FDA-approved medications with anti-tumor mechanisms that have been repurposed to complement conventional, standard of care (SOC) approaches.
This post reviews the current published evidence base for, and research activity in, the use of repurposed drugs in oncology. It culminates with a case series of five consecutive patients with metastatic lung cancer, all treated under the care of the same clinician (me) using similar combination repurposed drug therapies combined with metabolic interventions (i.e., ketogenic diets and/or fasting).
What makes the case series particularly remarkable is that four out of five patients showed no cancer progression over the observation period (one even achieved complete remission), despite advanced-stage diagnoses and advanced ages. Notably, two of the patients, per their insistence, achieved disease stability while receiving only repurposed drugs and dietary interventions – without any conventional chemotherapy, radiation, or targeted treatments.
I share these cases to offer a real-world glimpse into the potential of individualized, low-toxicity, evidence-informed repurposed drug protocols. This post will illustrate not only the scientific rationale behind drug repurposing in oncology but also the powerful role of patient engagement, metabolic interventions, and close clinical monitoring in the pursuit of disease control and quality of life.
Background
I previously wrote a post about how my interest in cancer was inspired by my close friend and co-founder of the FLCCC, Professor Paul Marik. His insight inspired me to do a deep dive into the history of research into the causes of cancer, culminating in the now validated “Metabolic Theory Of Cancer” (MTOC). I was further inspired by Paul’s comprehensive scoping review of the world’s medical literature, compiling the scientific and clinical evidence bases for dozens of repurposed drugs and nutraceuticals in the treatment of cancer. Paul’s work was an impressive effort, as it drew from nearly 1,500 scientific references. That work culminated in his increasingly popular book, now in its 2nd edition, called Cancer Care.
In a previous series, I explored the scientific evidence supporting the Metabolic Theory of Cancer (MTOC), which overturned the long-standing Somatic Mutation Theory (SMT) about 15 years ago, with the final pieces to the puzzle stemming from the groundbreaking work of Dr. Thomas Seyfried. His research helped fill the final gaps in the understanding of how cancer originates, offering a comprehensive framework that aligns with decades of observations dating back to Otto Warburg’s Nobel Prize–winning discovery in 1927—that cancer cells uniquely generate energy primarily through glucose fermentation, even in the presence of oxygen, unlike normal cells.
I hope it goes without saying that if the understanding of the “root cause” of a disease changes, so should the treatment approach. That is what we have done at the Leading Edge Clinic. Meanwhile, the field of oncology is still obsessively (and financially) focused on cytotoxic therapies (chemotherapy and radiation) rather than therapies targeting disrupted metabolic pathways.
However, our goal at Leading Edge is not to recommend that patients forego standard of care therapies (SOC) but instead that SOC should be “complemented” with more diverse, mechanistic, and non-toxic approaches, based on the considerable scientific and clinical data that Paul compiled.
Most significantly, in Paul’s scoping review and book, he graded the strength of the scientific evidence for repurposed drugs and nutraceuticals, where he identified only seventeen as having a “strong” level of evidence to support, eight as having a “weak level of evidence” to support, fifteen others which he deemed the evidence “insufficient” and another five as having evidence to “recommend against.”
Remarkably, Paul only found sufficient scientific (i.e., in vivo, in vitro, and clinical) evidence to make assessments on a total of 60 repurposed drugs and nutraceuticals, even though there are 254 repurposed drugs and over 2,000 nutraceuticals that purportedly have anti-tumor mechanisms. I often tell my patients that the “best” treatment for cancer might be in that dizzying list of potential candidates, but we don’t have the evidence of both safety and efficacy for me to recommend them.
My practice is to instead rigidly stick to the compounds with the “strongest published evidence” (although I have made exceptions with specific therapies that have had their research severely restricted, as per “The Kory Scale” (wink, wink).
This is important because many cancer patients, upon diagnosis, begin to research therapies online and on social media (often obsessively, but who can blame them). Once they start doing that, they find claims of efficacy and/or cure for… just about anything. As a result, they are constantly asking me about this therapy or that therapy that they have read about. I say, “I don’t know,” or, in cases where there is little published research, I typically recommend against it due to lack of safety data with chronic use (remember, first do no harm).
One example supporting the above concern is the widespread recommendations to use soursop fruit for its anti-cancer mechanisms, ignoring the fact that chronic use has been shown to cause Parkinson’s.
Again, I want to emphasize that we do not recommend that patients avoid or refuse well-established and evidence-based “standard of care” (SOC) approaches like chemotherapy, radiation, surgery, and targeted therapies. We insist that our patients have both an oncologist and a primary care provider, although to be fair, I can’t tell my patients what to do – a minority decline standard of care or “system docs” despite my recommendation that they work with one. Iatrophobia and nosocomephobia run rampant, it seems.
One — and only one — of the reasons I make this recommendation is, admittedly, a “defensive” one. If a patient experiences a poor outcome after choosing to rely solely on repurposed drug protocols, I want to avoid the risk of being sued by a family member who disagreed with that choice and later claims I failed to sufficiently encourage the patient to seek more established oncology guidance — a scenario that has, in fact, happened to other physicians who treated cancer patients with so‑called “alternative” methods.
Observational Study Of Repurposed Drugs In Cancer
Further, we applied for and obtained ethics oversight approval (IRB) to conduct a prospective, observational study to compare the survival and functional status of patients treated with complementary repurposed drug protocols versus patients treated with standard of care therapies alone.
That research study is just part of the work that my new non-profit, called Rebuild Medicine, is doing in advocacy for the research and use of repurposed medicines.
This post was inspired by the fact that I saw three of the five patients I am treating for metastatic lung cancer in the past few weeks, and was very pleased with their progress. Although the study is still ongoing (and will be for several years), I decided to write a preliminary, interim report of the metastatic lung cancer patients that I have personally treated, all presented consecutively, leaving none out.
I cannot overemphasize the importance of avoiding “cherry-picking” when discussing treatment outcomes, a practice that social media is riddled with – all you hear about are cases of cures by providers or patients without a minimum of effort (or ability) to compile data in an unbiased, transparent, systematic manner. This behavior runs the risk of overestimating the efficacy of such approaches, which might lead patients to avoid SOC, whose efficacy, although often sub-optimal or even harmful if not well chosen, is well established “scientifically.”
Growing Focus On Combination Repurposed Drug Therapy in Modern Oncology
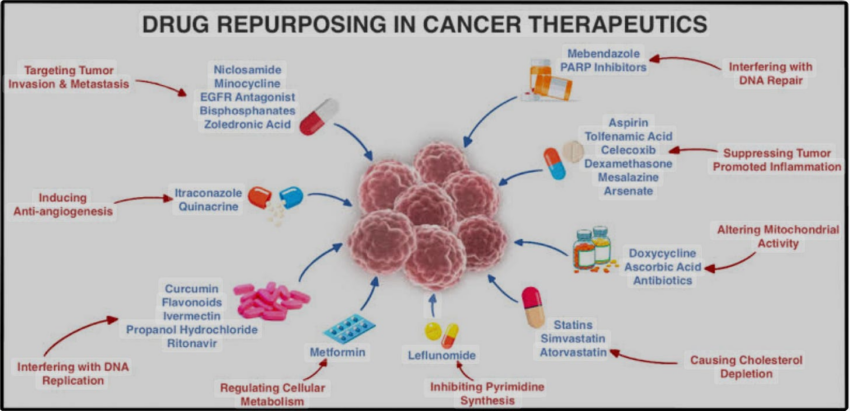
Treating cancer with combinations of repurposed drugs—medications originally developed for other indications—is no longer just an alternative approach; it is increasingly recognized as a mainstream strategy in oncology. Over the past decade, the number of major academic centers and research groups conducting rigorous clinical trials on repurposed drug protocols for cancer has grown dramatically, reflecting rising interest from both the scientific community and regulatory bodies.
The drug repurposing movement utilizes the central or ancillary attributes of a drug typically used for non-cancer indications to constructively interact with a cancer’s growth mechanisms, thereby slowing the cancer’s growth.
Know that such approaches, instead of directly killing cancer cells, simply target multiple, growth-driving pathways. On page 31 of Paul’s Cancer Care book, he lists the main pathways our Leading Edge Clinic targets with our combination regimens (Hexokinase 2, p53, TGF-B, Wnt, Notch, PI3/AKT, Hedgehog, IGF-1).
Two cancer non-profit organizations (The AntiCancer Fund and Global Cures) partnered to create The ReDO project (Repurposing Drugs in Oncology). ReDo created a database of all published, planned or active trials of repurposed drugs in cancer from U.S, European, and WHO Trial Registries. They identified 970 trials from 45 countries:
Current RCTs OF Repurposed Drugs in Oncology:
Now, although the ReDO project has identified 970 trials of repurposed drugs in oncology, very few are “actionable” given that many were terminated for lack of enrollment, others are still recruiting, or their recent status is unclear in the registry (updates not filed). Most disappointing is that the vast majority tested a single repurposed drug added to SOC (Ed: dumb). It is challenging to find published results of trials testing the addition of multiple agents at the same time; however, of the few available, the results are highly encouraging.
In one trial called CUSP9, they treated patients with a combination of 9 (yes, 9) different repurposed drugs in addition to standard of care. Note this approach is in line with our Leading Edge clinic practice and observational study, where we commonly use combinations of up to ten or more different therapies depending on stage and type of cancer (and “type” of patient).
GLIOBLASTOMA TRIALS
The majority of combination repurposed drug trials study patients with glioblastoma, a brain cancer. One reason is that glioblastoma is one of the more deadly cancers and has a highly predictable median overall survival of 15 months and a two-year survival of 27%, despite SOC combinations of surgery, chemotherapy, radiation, and oral maintenance chemotherapy.
This highly predictable (and terrible) survival allows for comparison in outcomes between the two approaches, as you will see below. Another reason is that glioblastoma has numerous mechanisms driving its growth, thus demanding a combination, multi-mechanistic approach. The results of the accumulating data are impressive:
CUSP9
Repurposed regimen: aprepitant, auranofin, captopril, celecoxib, disulfiram, itraconazole, minocycline, ritonavir, and sertraline.
A report of their phase 1 trial in 2021 showed:
➤ 30% of patients were alive and disease-free at over 4 years post-treatment.
-
Compared to historical prognosis with standard-of-care (SOC) therapies:
➤ Long-term disease-free survival (>4 years) is extremely rare,
➤ Typically occurs in under 5–10% of patients,
➤ Mostly limited to exceptional responders.
So, 30% of such patients were alive and disease-free over 4 years later, compared to only 5-10% being alive historically? Wow.
METRICS
Published in 2019, this study included 95 patients with Stage IV advanced glioblastoma who they treated with four drugs in addition to SOC (metformin, atorvastatin, mebendazole, and doxycycline). Check out the survival compared to historical controls:
➤ Two-year survival = 64% vs. 26-28%
➤ Median survival of repurposed patients = 27.1 months vs. 14-15 months
Again wow. Also, know that in this study, the median duration between diagnosis and start of the 4-drug regimen was 6.6 months. Imagine if they had started treatment at diagnosis instead of almost 7 months later? Further, 85% of patients tolerated all four drugs “without issue.”
CLOVA
Another four drug regimen study in glioblastoma patients (note the 4 drugs were completely different than the METRICS or CUSP9 studies above): cimetidine, lithium, olanzapine (an anti-psychotic?), and valproate. Results:
-
7 patients with recurrent, chemotherapy-resistant GBM
-
All were “RPA class 7.” RPA (recursive partitioning analysis) is a statistical tool used to stratify patients by expected survival. Class 7 refers to the group of patients with the poorest prognosis.
-
Median overall survival (OS) after recurrence: 11.2 months vs. 4.3–4.9 months in historical controls alone (p = 0.004).
-
See their chart below depicting survival curves and note the steep, plummeting trajectory of historical controls compared with the more staggered, prolonged survival of those treated with (just 4) repurposed drugs:
Solid Tumors
COMBAT
This was a study that included 74 children with advanced, refractory, or relapsed pediatric solid tumors, many heavily pretreated and with poor prognosis. They were then treated across three European academic medical centers with celecoxib, vitamin D, fenofibrate, and retinoic acid along with standard chemotherapy regimens. In a historical comparison of the high-grade sarcoma subgroup, they reported:
-
Median overall survival with COMBAT: 15.4 months vs. 3.9 months in historical controls
→ COMBAT-treated patients lived nearly 4 times longer (p = 0.001)
In summary, although I could find only four small to modestly sized studies using combinations of repurposed drugs, to date, all have shown significant improvements in survival and tolerance to medications.
Planned/Ongoing Trials
MDACT: is testing 6 drugs in colon, biliary, lung, and brain cancer. The six drugs are: celecoxib, dapsone, disulfiram, itraconazole, pyrimethamine, and telmisartan.
MEMMAT– includes children with recurrent medulloblastoma, ependymoma, or atypical teratoid/rhabdoid tumors being treated with thalidomide, celecoxib, and fenofibrate alongside conventional chemotherapies.
Proposed Trial Protocols
I also discovered a series of published papers in which the authors proposed multi-repurposed drug regimens to treat several cancers; however, to date (years later), these studies have still not been conducted. This suggests that publishing such “aspirational trial protocols” has become a cottage industry in oncology – resume padding anyone?
A reason for the lack of conducting such trials could be that there is no funding (all meds are off-patent, so no financial incentive). Again, this is something Rebuild Medicine is hoping to change. All study acronyms below are hyperlinked to the paper:
AVRO: glioblastoma – aprepitant, vortioxetine, roflumilast, olanzapine.
IPIAD– pancreatic cancer – irbesartan, pyrimethamine, itraconazole, azithromycin, and dapsone
OPALS – lung and glioblastoma – pyrimethamine, cyproheptadine, azithromycin, loratadine, and spironolactone.
EIS: glioblastoma: itraconazole, metformin, naproxen, pirfenidone, quetiapine rifampin
MTZ: glioblastoma- minocycline, telmisartan, and zoledronic acid
ADZT -glioblastoma – apremilast, dapsone, zonisamide, and telmisartan
Why Standard Therapies Fall Short: Cancer Stem Cells
Another critical aspect of the role of repurposed drugs in treating cancer is that they can target cancer stem cells, something that current SOC approaches fail at doing. Know that cancer stem cells (CSCs) were first identified in leukemia in the 1990s and a decade later in solid tumors, which represents, in my mind, a relatively recent discovery in “medical time.”
CSCs are a small, resilient subset of tumor cells driving growth, relapse, and metastasis (they make up between 0.01% to 2% of a tumor). Unlike the other fast-dividing cancer cells targeted by standard cytotoxic therapies (chemotherapy, radiation, immunotherapy), CSCs exhibit self-renewal, differentiation, and anti-apoptotic pathways, enabling them to survive within protective tumor microenvironments (e.g., hypoxic or inflammatory niches).
These properties make CSCs not only resistant to conventional treatments, but some (chemo and RT) have even been shown to promote CSC proliferation, leading to tumor recurrence inadvertently. For example, this below study’s title is not reassuring:
This study is even less reassuring, yikes:
Leading Edge Clinic’s Unique Approach
Thus, I believe that one critically important aspect of our treatment approach (where Leading Edge departs from the rest of the drug repurposing in oncology movement) is based on our specific targeting of cancer stem cells (CSCs) with repurposed drugs.
From Perplexity AI:
Currently, there are no FDA-approved therapies specifically and exclusively targeting cancer stem cells (CSCs) as a distinct class of treatment.
Since chemotherapy and radiation target rapidly dividing cells, shrinking tumors but sparing (or again, even inducing) slow-growing CSCs, it should come as no surprise that the biopharmaceutical industry is currently developing various CSC-targeted therapies (e.g., very costly monoclonal antibodies requiring infusion centers). Still, these remain inaccessible and as yet unproven.
The Role Of Repurposed Drugs Against Cancer Stem Cells
Although research into developing patented treatments directed at CSCs is underway, the beauty is that, since the discovery of CSCs, extensive in vitro studies have identified affordable, accessible drugs that inhibit CSC proliferation, suggesting the potential of cost-effective, readily available alternatives. At Leading Edge Clinic, we specifically target CSCs with the repurposed drugs that have been identified as having anti-CSC mechanisms, aiming to prevent relapse and improve outcomes.
Repurposed Drugs Targeting CSCs
Based on our research and clinical experience, we use, depending on the cancer and the patient:
-
Metformin, ivermectin, mebendazole, doxycycline (antibiotics with anti-CSC properties)
-
Curcumin, green tea extract, resveratrol, berberine (plant-based compounds)
-
Melatonin, vitamin D3, omega-3 fatty acids (nutritional supplements)
-
Aspirin, diclofenac, statins (atorvastatin), phosphodiesterase 5-inhibitors (anti-inflammatory and metabolic modulators)
Our CSC-focused strategy addresses a critical gap in standard care, leveraging affordable repurposed drugs to enhance patient outcomes without reliance on expensive, centralized systems. This aligns with our mission to empower patients and challenge profit-driven healthcare models.
I include the below forward to Paul’s book, written by our friend, colleague and mentor, the pseudonymous Dr. Justus R. Hope (I really like his tree/roots analogy re: CSC’s in the below graphic):
Metastatic Lung Cancer Case Series From The Leading Edge Clinic
In the published CLOVA study mentioned above, only seven patients were included. Here I present the outcomes in five consecutive patients. This is important because it is impossible to know the true efficacy of a therapy if the only data point being shared is a dramatically positive result without any sense of how many did not respond or were harmed (you can be sure that practitioners blasting treatment successes on social media never post about their adverse outcomes or non-responders).
Further, what is often underemphasized in such “reports” are the other, potentially contributory treatments or dietary changes. Thus, in this case series (and upcoming ones), I did the following:
-
The patients all presented consecutively, with the same stage and type of cancer (metastatic lung cancer in this series)
-
Patients were all treated by the same clinician (me)
-
All treatments employed, both SOC and repurposed, are detailed
-
Estimates of adherence to ketogenic diets/fasting are included
-
Medical records of imaging and stage, along with dates, are provided
-
Reports of intolerances or side effects to therapies are all documented
-
Clinical assessments of performance status over time are included
Of the five consecutive metastatic lung cancer patients I have treated with combination repurposed drug protocols and ketogenic diets:
-
2 received no other conventional treatments (no chemo, RT, targeted therapies etc) but not on my advice; this came out of their personal preference, both are octogenarians
-
2 received concomitant oral tyrosine kinase inhibitors
-
1 received multiple, aggressive conventional therapies – pneumonectomy, post-op RT to new metastases, and numerous cycles of various chemotherapy regimens
As you will see from this series, as well as from the above studies, it is clear to me that “complementary” repurposed drug protocols have considerable efficacy. Still, I do not feel the evidence is anywhere near sufficient to suggest that patients forego more supported treatments at this time.
I am putting the following case series behind a paywall because 1) I am submitting for publication, 2) I will pay the (up to $2,000) fee to make the article open access, 3) I promise to post the article to my free subscribers when that happens 4) I put an immense amount of effort into performing the detailed chart review writing up the cases for this series.
I wrote up two versions of the case series, one short, one long:
-
The longer, granularly documented version with concurrent commentary can be found at this link (10+ minute read).
-
A more concise, AI-assisted version can be found at this link (3-minute read)
If you can afford to, and find value in the time, research, and care I invest in crafting these posts (and Op-Ed’s) which aim to expose critically important truths about the safety and efficacy of a diverse set of medical therapeutics, please support my work with a paid subscription.
Click this link for the original source of this article.
Author: Pierre Kory, MD, MPA
This content is courtesy of, and owned and copyrighted by, https://pierrekory.substack.com and its author. This content is made available by use of the public RSS feed offered by the host site and is used for educational purposes only. If you are the author or represent the host site and would like this content removed now and in the future, please contact USSANews.com using the email address in the Contact page found in the website menu.