“Times of crisis and fear can call forth bad science, even science we know in retrospect to be unethical.”
Bill Clinton, Remarks on Accepting the Report of the Advisory Committee on Human Radiation Experiments, October 3, 1995 https://www.govinfo.gov/
“Times of crisis and fear can call forth bad science, even science we know in retrospect to be unethical.”
Bill Clinton, Remarks on Accepting the Report of the Advisory Committee on Human Radiation Experiments, October 3, 1995 https://www.govinfo.gov/content/pkg/PPP-1995-book2/html/PPP-1995-book2-doc-pg1530.htm
In a recent June 17, 2025, letter to Dr. Vinayak Prasad (Director of the FDA’s Center for Biologics Evaluation and Research), based on the recently changed recommendations for the COVID-19 vaccine for children, I questioned the recommendation for children who are immunocompromised, as well as requested clarification of the current benefit-risk assessment. I also questioned why children were still being included in clinical trials.
pting the Report of the Advisory Committee on Human Radiation Experiments, October 3, 1995 https://www.govinfo.gov/content/pkg/PPP-1995-book2/html/PPP-1995-book2-doc-pg1530.htm
In a recent June 17, 2025, letter to Dr. Vinayak Prasad (Director of the FDA’s Center for Biologics Evaluation and Research), based on the recently changed recommendations for the COVID-19 vaccine for children, I questioned the recommendation for children who are immunocompromised, as well as requested clarification of the current benefit-risk assessment. I also questioned why children were still being included in clinical trials.
https://dailyclout.io/an-evolving-understanding-of-benefit-risk-assessment-of-the-covid-vaccines-protecting-our-most-vulnerable/ https://www.npr.org/2025/06/13/nx-s1-5431935/rfk-hhs-covid-vaccine-schedule-faq
Today, I’ve just read Dr. Prasad’s May 16, 2025, CENTER DIRECTOR DECISIONAL MEMO in which he acknowledges the below regarding the benefit-risk assessment for COVID-19 vaccines in general [bold added]:
- “Even rare vaccination-related harms, both known and unknown, now have a higher chance of outweighing potential benefits in non-high-risk populations.”
- “Although the FDA monitors the safety of all vaccines through post-market surveillance, it is important to acknowledge times at which the potential for benefit from vaccination among non-high-risk individuals is small and poorly defined.”
- “Because the absolute potential for benefit among non-high-risk groups is minimal…” https://www.fda.gov/media/186905/download
The Declaration of Helsinki states: “…those incapable of giving consent must only be included if the research is likely to either personally benefit them or if it entails only minimal risk and minimal burden.” https://www.wma.net/policies-post/wma-declaration-of-helsinki/
Yet today there are still healthy (“non-high-risk”) infants and children in COVID-19 vaccine clinical trials for which the “potential for benefit… is minimal,” such as in this interventional trial in “participants” as young as 6 months old:

Based on recent changes in the FDA’s benefit-risk assessment, COVID-19 vaccine research involving “non-high-risk” infants and children should fall under the category of “no prospect of direct benefit to individual subjects” and “greater than minimal risk”:

If clinical trials of products with “no prospect of direct benefit to individual subjects” and “greater than minimal risk” continue in infants and children, the risk must represent no more than “a minor increase over minimal risk.”
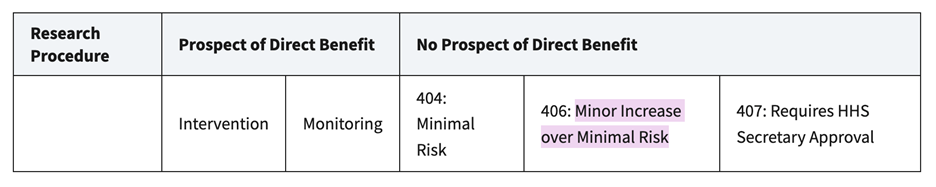
https://www.hhs.gov/ohrp/sachrp-committee/recommendations/2005-july-28-letter-appendix-b/index.html
Back on July 20, 2022, Dr. Peter Marks (Dr. Vinayak Prasad’s predecessor) had confirmed to me that, “FDA generally expects that [COVID-19 vaccine] pediatric trials would be initiated in specific age groups as soon as available data support that the vaccine would confer a prospect of direct benefit and acceptable risk to trial participants (21 CFR 50.52) [bold added].”

- Back then, the FDA’s stance was that the COVID-19 vaccines held the “prospect of direct benefit” for all children. Today, changes in their benefit-risk assessment suggest “no prospect of direct benefit” for those “non-high-risk.”
- Back then, the FDA’s stance was that the COVID-19 vaccines held “greater than minimal risk.” Today, the same is true.
Back then, pediatric COVID-19 vaccine trials were operating under the risk category of 21 CFR 50.52 in which there was “greater than minimal risk,” but also, the “prospect of direct benefit.” The risk was considered by the FDA justified by the anticipated benefit to the subject…
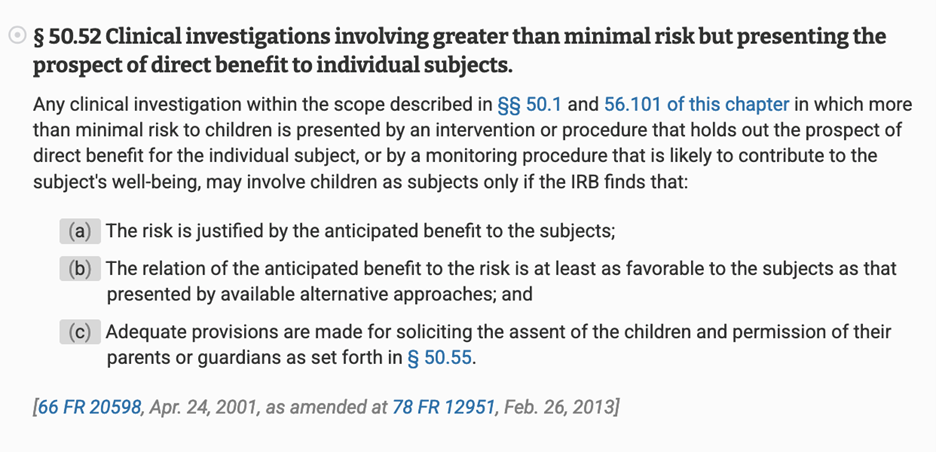
https://www.ecfr.gov/current/title-21/chapter-I/subchapter-A/part-50/subpart-D/section-50.52
Today, the risk category of 21 CFR 50.53 is more reflective of the changed benefit-risk assessment for those “non-high-risk”: there is (still) “greater than minimal risk,” but “no prospect of direct benefit.”

But in this scenario of no prospect of direct benefit, “greater than minimal risk” must be only “a minor increase over minimal risk.”
Also, the “intervention or procedure presents experiences to subjects that are reasonably commensurate with those inherent in their actual or expected medical, dental, psychological, social, or educational situations…,” i.e., interventions or procedures the children are “expected to experience” in their everyday lives. https://www.hhs.gov/ohrp/sachrp-committee/recommendations/2005-july-28-letter/index.html https://www.ecfr.gov/current/title-21/chapter-I/subchapter-A/part-50/subpart-D/section-50.53
What is “a minor increase over minimal risk”?
In HHS’ recommendations, “a minor increase over minimal risk” means “…potential harms associated with the procedure will be transient and reversible in consideration of the nature of the harm (restricted to time of procedure or short post-experimental period). [bold added].”
Myocarditis, alone, exceeds the “minor increase over minimal risk” category.

https://www.hhs.gov/ohrp/sachrp-committee/recommendations/2005-july-28-letter/index.html https://www.hhs.gov/ohrp/sachrp-committee/recommendations/2005-july-28-letter-appendix-b/index.html
An investigational pediatric trial should be halted (“clinical hold”) if it does not follow the following federal regulation: “Human subjects are or would be exposed to an unreasonable and significant risk of illness or injury…” https://www.ecfr.gov/current/title-21/chapter-I/subchapter-D/part-312/subpart-C/section-312.42 – p-312.42(b)(1)(i) Per FDA guidance, “Experimental interventions or procedures that present greater than low risk should offer a sufficient prospect of clinical benefit to justify exposure of a pediatric population to such risk.” https://www.fda.gov/media/101398/download
At age 19, in addition to surgery, I received radioactive iodine therapy (I-131) for thyroid cancer with lymph node metastases. My cancer journey planted the seeds of passion for a career in medicine, specifically, drug development. But in recent years, delving further into the history of medical experimentation, I’ve become horrified by the unethical I-131 and other radioisotope experiments performed by my own government on healthy vulnerable (and other) populations starting in the late 1940s (and continuing through 1990 based on the referenced report). Multiple studies, such as the Uptake of Iodine-131 in Normal Newborn Infants in Iowa City, were supported by such institutions as the U.S. Atomic Energy Commission and the American Cancer Society, and had results published in such journals as Pediatrics, AMA American Journal of Diseases of Children, and Science. Some radioisotope experimentation took place at The Argonne Cancer Research Hospital which was then operated by the University of Chicago. I received my I-131 treatment at the University of Chicago in 1980. https://www.lib.uchicago.edu/e/scrc/findingaids/view.php?eadid=ICU.SPCL.UCHOSPITALS&lang=en https://ehss.energy.gov/ohre/roadmap/roadmap/part3.html
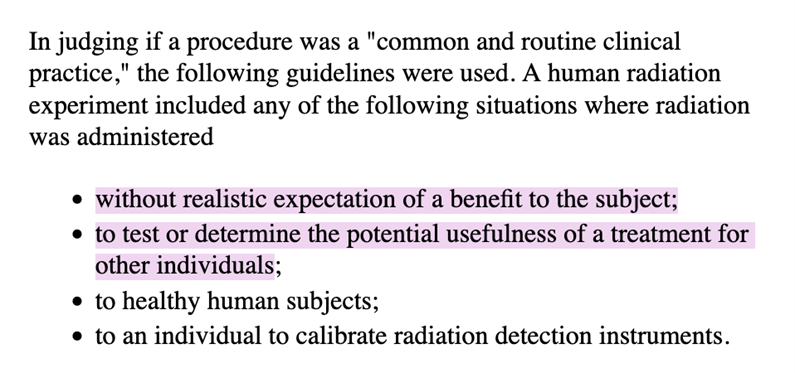
https://ehss.energy.gov/ohre/roadmap/roadmap/part3.html
I am the “other individual[s].”
It is a start that COVID-19 vaccines are finally acknowledged to be without realistic expectation of a benefit to “non-high-risk” children. The time to halt these trials in healthy children is now, and one next step in stopping them for all children.
We can’t let clinical trial history repeat itself.
The post Halting COVID-19 “Vaccine” Trials in the “Non-high-risk” When Benefit is Acknowledged as “Minimal”: A Next Step to Protecting All Children appeared first on DailyClout.
Click this link for the original source of this article.
Author: Sean Probber
This content is courtesy of, and owned and copyrighted by, https://dailyclout.io and its author. This content is made available by use of the public RSS feed offered by the host site and is used for educational purposes only. If you are the author or represent the host site and would like this content removed now and in the future, please contact USSANews.com using the email address in the Contact page found in the website menu.








