By
A Documented History of Corruption
In 2000, the U.S. House Committee on Government Reform published a landmark report confirming that the CDC’s vaccine advisory panel was deeply compromised by industry influence.
The committee uncovered systemic, ongoing conflicts of interest within both the ACIP and the FDA’s vaccine advisory counterpart:
-
7 out of 10 members of ACIP’s rotavirus working group—which drafted the recommendation for the Rotashield vaccine—had direct financial conflicts of interest.
-
Every ACIP member was granted a conflict-of-interest waiver, regardless of industry ties.
-
Members were allowed to vote on vaccine recommendations while holding stock in manufacturers, receiving grants, or serving as paid consultants.
-
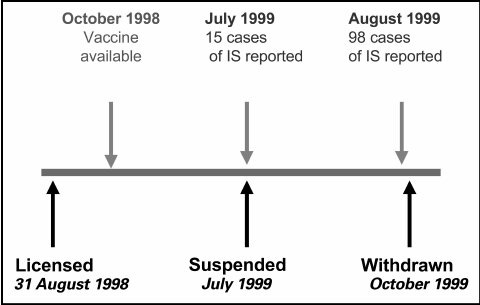
RotaShield® was taken off U.S. market in 1999. ACIP voted on October 22, 1999 to no longer recommend use of the RotaShield® vaccine for infants because of an association between the vaccine and intussusception.
In sworn testimony, a CDC ethics official admitted:
“We generally give [waivers] to everyone… we give them out freely.”
Named: The Most Compromised ACIP Members
Among the worst examples:
-
Dr. Paul Offit — Participated in the ACIP’s rotavirus working group while holding a patent on a competing Merck rotavirus vaccine. He also received a $350,000 grant from Merck and served as a Merck consultant, yet still voted on recommendations that would eventually benefit his product.
-
Dr. John Modlin — Then-Chair of ACIP, who owned 600 shares of Merck stock while voting on rotavirus policy. He also chaired the rotavirus working group that produced the recommendation.
-
Dr. Marie Griffin and Dr. Fernando Guerra — Both had Merck ties through funding, consulting, or grants, yet participated fully in committee decisions.
The Rotashield Disaster
In 1998, ACIP recommended Wyeth’s Rotashield vaccine for routine use in infants. Just 13 months later, the vaccine was pulled from the market after it was linked to a wave of serious bowel obstructions (intussusception) in children.
The U.S. House Committee on Government Reform report revealed:
-
ACIP members with conflicts of interest drafted the recommendation in private working groups, shielded from public oversight.
-
ACIP voted to recommend the vaccine before the FDA even approved it.
-
The committee that approved it was stacked with individuals financially tied to competing vaccine developers.
ACIP has not functioned as a true scientific body. For decades, it has served as an automated rubber stamper operated by the Bio-Pharmaceutical Complex. Since 2020, this troubling reality has only been reinforced by ACIP’s reckless recommendations of COVID-19 mRNA shots, which have resulted in mass injury and death.
For the first time in a generation, there is hope that U.S. vaccine policy may finally be shaped by independent experts who put public safety before profit.
If you’re curious as to what an ACIP meeting can look like, I recommend that you watch the following clip from 2018 where they rubber-stamp the Hepatitis B vaccine:
Epidemiologist and Foundation Administrator, McCullough Foundation
www.mcculloughfnd.org
Please consider following both the McCullough Foundation and my personal account on X (formerly Twitter) for further content.