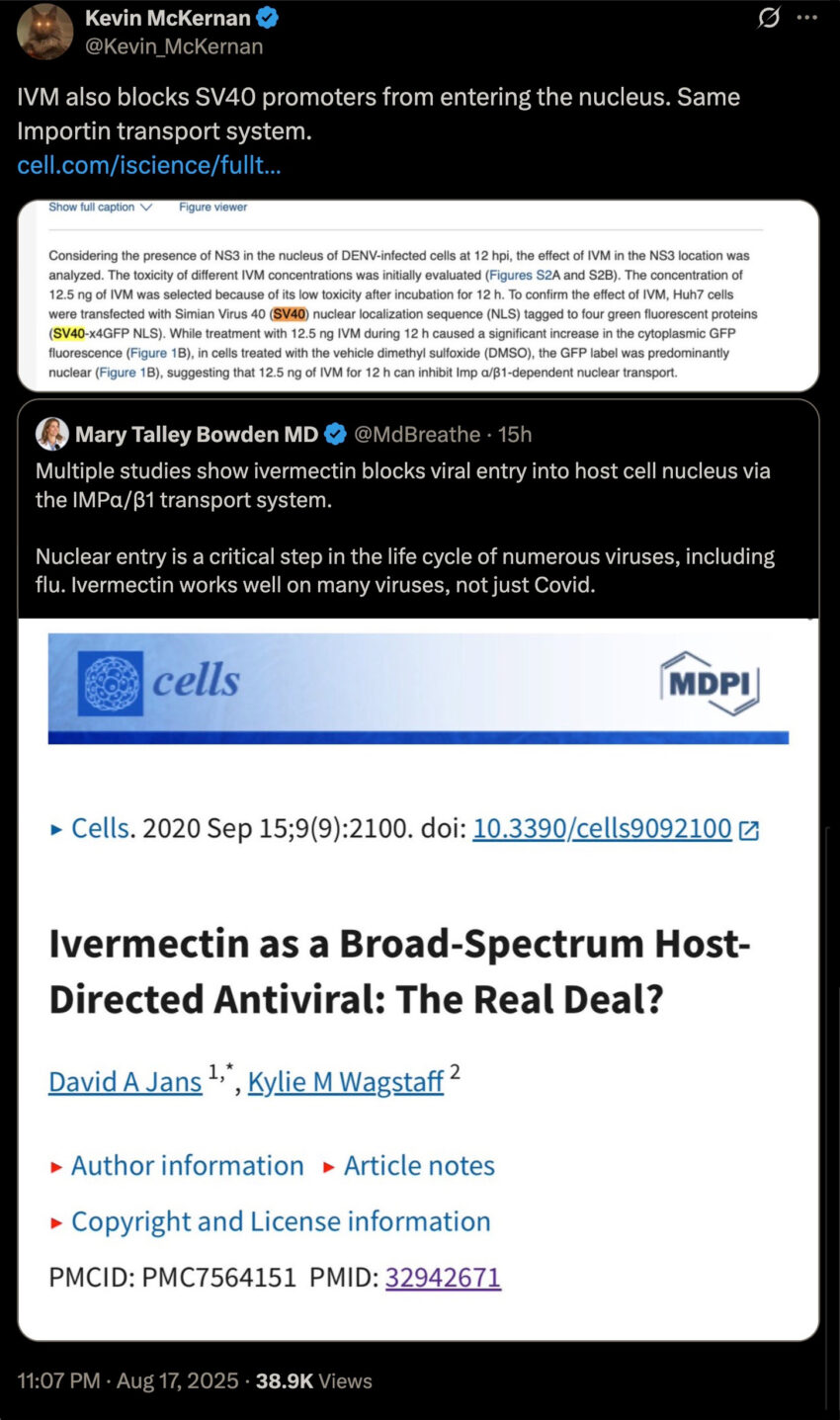
This is an important and potentially lifesaving update to the following article…
…because we now know that Ivermectin blocks the genetic sequence found in Pfizer’s Modified mRNA slow kill bioweapon “vaccine,” thus potentially preventing the highly carcinogenic SV40 promotor sequence from integrating into the cell’s host nucleus:
This is absolutely massive, because according to the cited research study entitled, An ivermectin – atorvastatin combination impairs nuclear transport inhibiting dengue infection in vitro and in vivo, we have proof that Ivermectin doesn’t only prevent (lab engineered) mosquito-born viruses like dengue (DENV), but it also inhibits the integration of SV40 promotor sequences into the cells.
And just like in yesterday’s article…
…it was surmised that unlike the DeathMist™ flu vaccine, Ivermectin would actually be an effective approach for preventing and treating seasonal flu, here is more proof that this is in fact the case:
Yes, influenza virus has proteins that are actively transported into the nucleus using host nuclear import machinery, including importin-α (Importin A).
Here’s a breakdown:
🦠 Influenza and Nuclear Import
✅ Influenza is unusual among RNA viruses in that it replicates its genome inside the nucleus of host cells.
This requires its viral components—particularly the ribonucleoprotein complexes (vRNPs)—to cross the nuclear envelope.
📦 Key Influenza Proteins That Use Importin-α
1. NP (Nucleoprotein)
NP contains a nuclear localization signal (NLS).
It interacts directly with importin-α, which then binds importin-β to form the import complex for nuclear entry.
This is essential for importing the vRNPs into the nucleus. 2. PA, PB1, PB2 (polymerase complex subunits) All three have been shown to carry non-canonical NLSs.
PB2 in particular has a well-characterized NLS that binds importin-α isoforms.
Specificity for importin-α isoforms varies between avian and human-adapted influenza strains (e.g., importin-α3 vs α7) and is associated with host range restriction. 3. NS1 (Nonstructural Protein 1) NS1 also shuttles into the nucleus and contains a classical NLS that interacts with importin-α.
Nuclear localization of NS1 helps it antagonize host mRNA processing and immune responses.
🧬 Mechanism: Importin-Mediated Nuclear Transport NLS-bearing viral protein binds importin-α.
Importin-α binds importin-β to form a ternary complex.
The complex docks at the nuclear pore complex (NPC) and translocates into the nucleus.
Inside, Ran-GTP dissociates the complex, releasing the viral protein.
🧪 Supporting Studies
Melen et al., 2007 (J Biol Chem): Showed PB2 uses multiple importin-α isoforms.
Resa-Infante et al., 2008 (Virology): Detailed role of importin-α in NP transport.
Boivin and Hart, 2011 (PLoS Pathogens): Highlighted how importin specificity impacts host adaptation and pathogenicity.
📌 Summary
Protein NLS? Uses Importin-α? NP Yes ✅ Strongly PB2 Yes ✅. Host-specific preference PB1/PA Yes ✅ To lesser degree NS1 Yes ✅. For immune evasion M1 Indirectly involved Possibly contributes to export Let me know if you want protein sequences with their NLS motifs or details on how this import process influences species tropism or vaccine design.
In conclusion, Ivermectin inhibits viral as well as SV40 promotor sequence replication by interfering with the nuclear import of viral proteins, and functions as a powerful antiviral by impeding the importin α/β1 replication pathway.
Ivermectin may very well be a prophylaxis against vaccine shedding as well.
In terms of the SV40 promotor sequence integration into the human genome post “vaccination,” it is unlikely that Ivermectin can undo this genetically modifying medical intervention, but we can certainly extrapolate that it may prevent and cure the various associated adverse events such as turbo cancer and other VAIDS symptoms.
Do NOT comply.
Click this link for the original source of this article.
Author: 2nd Smartest Guy in the World
This content is courtesy of, and owned and copyrighted by, https://2ndsmartestguyintheworld.substack.com and its author. This content is made available by use of the public RSS feed offered by the host site and is used for educational purposes only. If you are the author or represent the host site and would like this content removed now and in the future, please contact USSANews.com using the email address in the Contact page found in the website menu.