Editor’s note: This essay is too long for most email systems so please click on the headline to read the full piece on the Substack site. The Substack app has an audio reader built in if you want to listen to an article instead of reading it. If you need access to any of the articles listed below that are behind a paywall, try Sci-hub, it’s free and works pretty well. This article is a heavy lift, but I believe it rewards a careful reading. Stay tuned for Part II in the next few days.
I. Introduction
Evidence-Based Medicine (EBM) is a relatively recent phenomenon. The term itself was not coined until 1991. It began with the best of intentions — to give frontline doctors the tools from clinical epidemiology to make science-based decisions that would improve patient outcomes. But over the last three decades, EBM has been hijacked by the pharmaceutical industry to serve the interests of shareholders rather than patients. Today, EBM gives preference to epistemologies that favor corporate interests while instructing doctors to ignore other valid forms of knowledge and their own professional experience. This shift disempowers doctors and reduces patients to objects while concentrating power in the hands of pharmaceutical companies. EBM also leaves doctors ill-equipped to respond to the autism epidemic and unable to produce the sorts of paradigm-shifts that would be necessary to address this crisis.
In this article I will:
-
provide a brief history of EBM;
-
explain how evidence hierarchies work;
-
explore ten general and technical criticisms of EBM and evidence hierarchies;
-
examine the American Medical Association’s 2002, 2008, and 2015 evidence hierarchies;
-
highlight the corporate takeover of EBM; and
-
explore the implications of these dynamics for the autism epidemic.
II. History of Evidence-Based Medicine
Medicine faces the same challenges as any other branch of knowledge — deciding what is “true” (or at least “less wrong”). Since its emergence in 1992, EBM has become the dominant paradigm in the philosophy of medicine in the United States and its impact is felt around the world (Upshur, 2003 and 2005; Reilly, 2004; Berwick, 2005; Ioannidis, 2016). Through the use of evidence hierarchies, EBM privileges some forms of evidence over others.
Hanemaayer (2016) provides a helpful genealogy of EBM. Epidemiology — “the branch of medical science that deals with the incidence, distribution, and control of disease in a population” — has been a recognized field for hundreds of years. But clinical epidemiology, defined as “the application of epidemiological principles and methods to problems encountered in clinical medicine” first emerged in the 1960s (Fletcher, Fletcher, and Wagner, 1982). Feinstein (1967) is credited as the catalyst for the emergence and growth of this new discipline. Feinstein, in his book Clinical Judgment (1967) wrote, “Honest, dedicated clinicians today disagree on the treatment for almost every disease from the common cold to the metastatic cancer. Our experiments in treatment were acceptable by the standards of the community, but were not reproducible by the standards of science.” So Feinstein proposed a method for applying scientific criteria to clinical judgements in clinical situations.
According to Hanemaayer (2016), around the same time, David Sackett was leading the first department of clinical epidemiology at McMaster University in Canada. Sackett was influenced by Feinstein and trained an entire generation of future doctors in clinical epidemiology. In the 1970s, Archibald Cochrane expanded the use of randomized controlled trials to a broader range of medical treatments. In 1980, the Rockefeller Foundation funded the International Clinical Epidemiology Network (INCLEN) which took the methods and philosophy of clinical epidemiology worldwide. The efforts of INCLEN would later receive the support of the U.S. Agency for International Development, the World Health Organization, and the International Development Research Centre.
Various terms have been used to describe the methods of clinical epidemiology. Eddy (1990) used the term “evidence-based.” At about the same time the residency coordinator at McMaster University, Dr. Gordon Guyatt, was referring to this growing discipline as “scientific medicine” but apparently this term never caught on with the residents (Sur and Dahm, 2011). Eventually Guyatt settled on the term “evidence-based medicine” in an article in 1991 (Sur and Dahm, 2011).
An Evidence-Based Medicine Working Group (EBMWG) was formed, comprised of 32 medical faculty members mostly from McMaster University but also from universities in the United States. In 1992, the EBMWG planted a flag for their particular approach to the philosophy of medicine with an article in JAMA titled, “Evidence-Based Medicine: A New Approach to Teaching the Practice of Medicine.” The article reads less like a traditional scientific journal article and more like a political manifesto. In the first paragraph they announced their intention to supplant the traditional practices of doctors with the methods and results from clinical epidemiology.
A NEW paradigm for medical practice is emerging. Evidence-based medicine de-emphasizes intuition, unsystematic clinical experience, and pathophysiologic rationale as sufficient grounds for clinical decision making and stresses the examination of evidence from clinical research. Evidence-based medicine requires new skills of the physician, including efficient literature searching and the application of formal rules of evidence [in] evaluating the clinical literature (EBMWG, 1992).
The article mostly consists of recommendations to consult the epidemiological literature following “certain rules of evidence” which are not defined before making any clinical decision (EBMWG, 1992). The authors also provide an evaluation form for “more rigorous evaluation of attending physicians” based on how consistently they “substantiate decisions” by consulting the medical literature (EBMWG, 1992). But the important point was not the steps per se, but who had ultimate decision-making authority within the medical profession. The EBMWG (1992) article was an announcement that henceforth, clinical epidemiology was at the top of the authority pyramid (what remains to be explained is why doctors fell in line). Over the next ten years the EBMWG published twenty-five articles on EBM in JAMA (Daly, 2005).
Many have questioned the tone and approach of the early EBMWG vanguard (see: Upshur, 2005; Goldenberg, 2005; and Stegenga, 2011 and 2014). But the article, along with extensive organizing within the medical community, had the desired effect. EBMWG (1992) has since been cited over 6,900 times and EBM has become hegemonic throughout medicine — thoroughly reshaping the practices of doctors, clinics, medical schools, hospitals, and governments.
In 1994, Sackett left McMaster University to start the Centre for Evidence-Based Medicine at Oxford University which quickly became a dominant force in the EBM movement (Hanemaayer, 2016). Sackett et al. (1997) systematized EBM to include the following five steps:
-
Formulate an answerable question;
-
Track down the best evidence of outcomes available;
-
Critically appraise the evidence (i.e. find out how good it is);
-
Apply the evidence (integrate the results with clinical expertise and patient values); and
-
Evaluate the effectiveness and efficiency of the process (to improve next time).
So far so good, but the Devil is always in the details.
III. Evidence Hierarchies
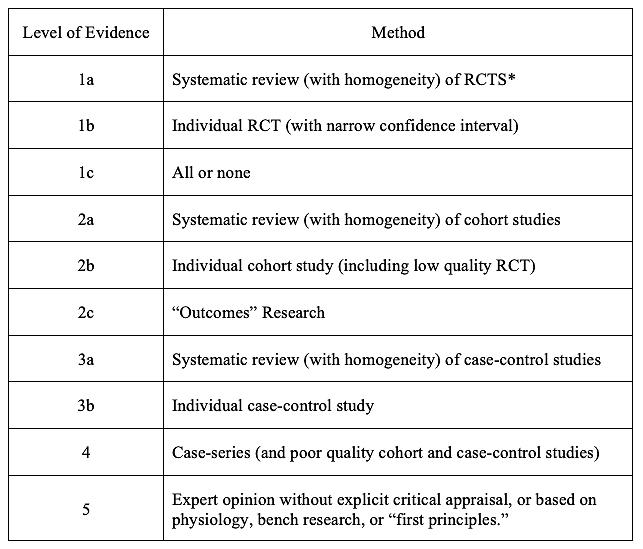
At first glance EBM appears straightforward and helpful. Problems appear once one tries to operationalize it. At the heart of evidence-based medicine are evidence hierarchies (Stegenga, 2014). Evidence hierarchies, as the name suggests, are categorical rankings that give preference to some ways of knowing over others. Rawlins (2008) found that 60 different evidence hierarchies had been developed as of 2006. Some of the best known evidence hierarchies include the Oxford Centre for Evidence-Based Medicine (CEBM), the Scottish Intercollegiate Guidelines Network (SIGN), and the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) (Stegenga, 2014).
For the purposes of this initial discussion I will focus on the Oxford CEBM because it was the first in widespread use and it is representative of the larger field (Stegenga, 2014).
Table 1, simplified version of Evidence Hierarchy, Oxford Centre for Evidence-Based Medicine last updated by Howick, 2009:
*The CEBM definition of “systematic reviews” sometimes includes meta-analysis.
Source: Stegenga (2014). Available from the Centre for Evidence-Based Medicine (2009).
In theory, EBM and evidence hierarchies could be two separate things. In practice, evidence hierarchies are how one “critically appraises the evidence” — Step 3 in Sackett et al. 1997 described above (Stegenga, 2014).
IV. Ten general and technical criticisms of evidence-based medicine and evidence hierarchies
In this section I will review ten general and technical criticisms of EBM. The arguments are that:
1. EBM has become hegemonic in ways that crowd out other valid forms of knowledge;
2. Evidence hierarchies do not just sort data, they legitimate some forms of data and invalidate other forms of data;
3. Meta-analyses and systematic reviews of RCTs are beset with epistemic problems;
4. Most RCTs are designed to identify benefits but they are not the proper tool for identifying harms;
5. RCTs are designed to address selection bias but other forms of bias remain;
6. Case reports and observational studies are often just as accurate as RCTs;
7. EBM is not based on evidence that it improves health outcomes;
8. EBM and evidence hierarchies reflect authoritarian tendencies in medicine;
9. Evidence hierarchies have reshaped the practice of medicine for the worse; and
10. Evidence hierarchies objectify and/or overlook patients.
1. EBM has become hegemonic in ways that crowd out other valid forms of knowledge.
There is widespread agreement that EBM has become the dominant paradigm in clinical medicine. Upshur (2005) writes:
Now virtually every dimension of health care — from nursing to mental health care to policy making to humanitarian medical intervention — is striving to become evidence-based. PubMed currently has over 20,000 citations to “evidence-based.”
Reilly (2004) is unconcerned with EBM’s shortcomings and unequivocal in assessing its dominance in medicine today (this passage is flagged by critics of EBM including Goldenberg, 2009, and Stegenga, 2014 for its stridency):
Few would disown the EBM hypothesis — providing evidence-based clinical interventions will result in better outcomes for patients, on average, than providing non-evidence-based interventions. This remains hypothetical only because, as a general proposition, it cannot be proved empirically. But anyone in medicine today who does not believe it is in the wrong business (Reilly 2004).
Berwick (2005) provides a history of the promising early origins of EBM but then warns that things have gone too far. He writes:
…we have overshot the mark. We have transformed the commitment to “evidence-based medicine” of a particular sort into an intellectual hegemony that can cost us dearly if we do not take stock and modify it. And because peer reviewed publication is the sine qua non of scientific discovery, it is arguably true that hegemony is exercised by the filter imposed by the publication process…
Berwick (2005) then draws attention to common sense ways of knowing such as practice, experience, and curiosity, that are excluded by EBM (I love this quote!):
How much of the knowledge that you use in your successful negotiation of daily life did you acquire from formal scientific investigation — yours or someone else’s? Did you learn Spanish by conducting experiments? Did you master your bicycle or your skis using randomized trials? Are you a better parent because you did a laboratory study of parenting? Of course not. And yet, do you doubt what you have learned?
Far from setting doctors free to practice their craft at the highest level, Berwick (2005) sees EBM as encouraging doctors to exclude valuable ways of knowing:
… the very success of the movement toward formal scientific methods that has matured into the modern commitment to evidence-based medicine now creates a wall that excludes too much of the knowledge and practice that can be harvested from experience, itself, reflected upon.
2. Evidence hierarchies do not just sort data, they legitimate some forms of data and exclude other forms of data.
Although EBM in the early years made reference to the totality of evidence, soon EBM became a way of excluding all studies except double-blind, randomized, controlled trials (RCTs) from the analysis. Stegenga (2014) writes: “The way that evidence hierarchies are usually applied is by simply ignoring evidence that is thought to be lower on the hierarchies and considering only evidence from RCTs (or meta-analyses of RCTs).”
Often this is not just implicit but explicit:
In an article which purported to provide the best way to distinguish effective medical interventions from those which are ineffective or harmful, the article stated that one should “discard at once all articles on therapy that are not about randomized trials” (Department of Clinical Epidemiology and Biostatistics, 1981, in Stegenga, 2014).
Strauss et al. (2005), in a textbook on the practice and teaching of EBM, also suggests that some forms of evidence can be discarded:
If the study wasn’t randomized, we suggest that you stop reading it and go on to the next article in your search. (Note: We can begin to rapidly critically appraise articles by scanning the abstracts to determine if the study is randomized; if it isn’t, we can bin it.) Only if you can’t find any randomized trials should you go back to it (Strauss et al. 2005 in Borgerson, 2009).
3. Meta-analyses and systematic reviews of RCTs are beset with epistemic problems.
Meta analyses of RCTs and/or systematic reviews of RCTs are consistently at the top of most evidence hierarchies. The concept of aggregating the findings from several studies seems unassailable. But understanding how it works in practice reveals that it has the appearance of accuracy and objectivity only by eliding the subjectivity at the core of the technique. Meta-analyses tend to treat evidence as a commodity like wheat, copper, or sugar that just needs to be sorted and weighed. Stegenga (2011) explains that:
Meta-analysis is performed by (i) selecting which primary studies are to be included in the meta-analysis, (ii) calculating the magnitude of the effect due to a purported cause for each study, (iii) assigning a weight to each study, which is often determined by the size and the quality of the study, and then (iv) calculating a weighted average of the effect magnitudes (p. 498)…. [B]y pooling data from multiple studies the sample size of the analysis increases, which tends to decrease the width of confidence intervals, thereby potentially rendering estimates of the magnitude of an intervention effect more precise, and perhaps statistically significant.
While meta-analysis aims for greater objectivity, in fact, it is still a subjective exercise. Stegenga (2011) writes, “Epidemiologists have recently noted that multiple meta-analyses on the same hypotheses, performed by different analysts, can reach contradictory conclusions.”
Furthermore, many meta-analyses are plagued by the same financial conflicts of interest as RCTs and other ways of gathering evidence:
Barnes and Bero (1998) performed a quantitative assessment of multiple meta-analyses which reached contradictory conclusions regarding the same hypothesis, and found a correlation between the outcomes of the meta-analyses and the analysts’ relationships to industry…. In another example, there have been 124 meta-analyses of antihypertensive drugs. Meta-analyses of these drugs were five times more likely to reach positive conclusions regarding the drugs if the reviewer had financial ties to a drug company (Yank, Rennie, & Bero, 2007 in Stegenga, 2011).
Meta-analyses are not nearly as precise as their proponents would have one believe.
Different weighing schemes can give contradictory results when evidence is amalgamated. An empirical demonstration of this was given by Jüni, Witschi, Bloch, and Egger (1999). They amalgamated data from 17 trials testing a particular medical intervention, using 25 different scales to assess study quality (thereby effectively performing 25 meta-analyses)…. Their results were troubling: the amalgamated effect sizes between these 25 meta-analyses differed by up to 117% — using exactly the same primary evidence. The authors concluded that “the type of scale used to assess trial quality can dramatically influence the interpretation of meta-analytic studies” (Jüni et al. 1999 in Stegenga, 2011).
Meta-analyses also suffer from low inter-rater reliability.
Not only does the choice of quality assessment scale dramatically influence the results of meta-analysis, but so does the choice of analyst. A quality assessment scale known as the ‘”risk of bias tool” was devised by the Cochrane group to assess the degree to which the results of a study “should be believed.” Alberta researchers distributed 163 manuscripts of RCTs among five reviewers, who assessed the RCTs with this tool, and they found the inter-rater agreement of the quality assessments to be very low (Hartling et al., 2009). In other words, even when given a single quality assessment tool, and training on how to use it, and a narrow range of methodological diversity, there was a wide variability in assessments of study quality (Stegenga, 2011).
It is not that subjectivity itself is necessarily a problem. The subjective wisdom that comes from years of experience could be quite helpful in evaluating the evidence. The problem with meta-analyses as currently practiced is that those involved usually do not acknowledge their own subjectivity while simultaneously excluding the sort of reasoned subjective analysis (from doctors, patients, or perhaps even philosophers) that might be helpful. Indeed, meta-analyses as currently practiced leave out the political and economic contextual factors that are likely to corrupt a study’s results:
A good review is based on intimate personal knowledge of the field, the participants, the problems that arise, the reputation of different laboratories, the likely trustworthiness of individual scientists, and other partly subjective but extremely relevant considerations. Meta-analysis rules out any such subjective factors (Eysenck, 1994 in Stegenga, 2011).
Stegenga (2011) concludes, “the epistemic prominence given to meta-analysis is unjustified.”
4. Most RCTs are designed to identify benefits but they are not the proper tool for identifying harms.
Upshur (2005) writes that:
RCTs can serve potent economic interests, and the ascendancy of the randomized trial as the most reliable form of evidence detracts from considering other, equally cogent forms of evidence as informative or having standing in debates about the safety and harm of treatments. RCTs are also parsimoniously powered to get answers efficiently, usually in the shortest period of time, largely because the pharmaceutical company sponsoring the trial needs the data for regulatory approval…. So RCTs and meta-analysis are underpowered to tell us what the likely accurate harm/benefit ratio of therapy is, and they are conducted and, once available, become “evidential” on populations for the most part quite unlike those that take the drugs, often with significant co-morbidity burdens. Therefore, we actually do not have the full “evidence” of what a medication is capable of doing on the basis of RCTs alone.
Michael Rawlins chaired the Committee on the Safety of Medicines (UK) from 1992 to 1998 and was the founding chair of the National Institute for Clinical Excellence from 1999 to 2013. From 2012 to 2014 he was President of the Royal Society of Medicine and in 2014 served as chair of the Medicines and Healthcare products Regulatory Agency (roughly the equivalent of the medical portion of the U.S. Food & Drug Administration). Rawlins (2008) writes,
RCTs are designed to ensure that the statistical power will be sufficient to demonstrate clinical benefit. Such power calculations do not, however, usually take harms into account (Evans 2004). As a consequence, although RCTs can identify the more common adverse reactions, they singularly fail to recognise less common ones or those with a long latency (such as malignancies). Most RCTs, even for interventions that are likely to be used by patients for many years, are only of six- to 24-months duration. And, if adverse events are detected at a statistically significant level, it is easy to dismiss them as being due to chance rather than a real difference between the groups (Rawlins, 2008).
Rawlins (2008) concludes that “only observational studies can offer the evidence required for assessing less common, or long-latency, harms.”
Stegenga (2014) writes,
The vast majority of RCTs in medical research maximize the power to detect benefit at the expense of the power to detect harm. The majority of serious harms caused by medical interventions are detected by so-called Phase IV post-approval studies, which are almost always limited to observational analyses of anecdotal clinical reports. Thus, for hypotheses of this kind this intervention causes harm, medical research is limited to evidence from methods not typically placed near the top of mainstream evidence hierarchies…
Stegenga (2016) deepens his critique of how RCTs fail to detect harms:
Most evidence regarding the harms of medical interventions is generated by studies which are funded and controlled by the manufacturers of the interventions under investigation, and whose interests are best-served by underestimating the harm profile of such interventions. This leads to widespread limitation of the evidence regarding harms that is made available to independent scientists and policy-makers, and this, in turn, contributes to the underestimation of the harm profiles of medical interventions. Regulators lack the authority to properly estimate harm profiles of medical interventions, and frequently contribute to shrouding the relevant evidence regarding harms in secrecy (Stegenga, 2016).
Like Upshur (2005) and Rawlins (2008), Stegenga (2016) points out that most RCTs are just long enough to detect benefits but often not long enough to detect harms and the size of the trial is usually calculated to achieve statistical significance for obvious benefits while not being large enough to capture “severe but rare” harms. But he also points out ways that RCTs are intentionally manipulated to produce desired outcomes:
To maximize the observed effect size and minimize the variability of data, trial designers employ various criteria constraining what subjects are included or excluded from the trial…. The most egregious of these trial design features are called “enrichment strategies”: after the enrollment of subjects, but prior to the start of data collection, subjects are tested for how they respond to placebo or the experimental intervention, and those subjects that do well on placebo or (and sometimes and) those subjects that do poorly on the experimental intervention are excluded from the trial (Stegenga, 2016).
FDA post-market surveillance is under-funded by design and not sufficiently staffed to respond to the size of the task. Stegenga (2016) points out the ways that EBM contributes to make the problem worse:
There is strong reason to think that post-market passive surveillance severely underestimates harms of medical intervention. One empirical evaluation of this puts the underestimation rate at 94% (this was based on a wide-ranging empirical survey by Hazell and Shakir 2006). Unfortunately, because observational studies and passive surveillance do not involve a randomized design, they are typically denigrated relative to randomized controlled trials…. Since most evidence regarding harms of medical interventions comes from non-randomized studies (especially rare severe harms), the dominant view of the evidence-based medicine (EBM) movement thereby denigrates the majority of evidence regarding harms of medical interventions (Stegenga, 2016, p. 495).
Stegenga (2016) concludes that, “Because harms of medical interventions are systematically underestimated at all stages of clinical research, policy-makers and physicians generally cannot adequately assess the benefit-harm balance of medical interventions.” Systematically underestimating harms, lack of adequate information for regulatory decisions, and insufficient funding for post-market surveillance are a reflection of the power of pharmaceutical companies to shape the regulatory and political environment.
5. RCTs are designed to address selection bias but other biases remain.
Upshur and Tracy (2004) write that the purpose of randomized trials is to minimize selection bias. However, they note, “this leaves undisturbed concerns about affluence bias, that is, the ability of certain interests to purchase and disseminate evidence; or the relevance bias, that is, the ability of interests to set the evidence agenda” (Upshur and Tracy, 2004).
The high cost of RCTs means that there are only certain actors who are able to engage in this sort of research — usually pharmaceutical companies and academics working under large government grants. Rawlins (2008) points out that the median cost of an RCT in 2005-2006 in the U.K. was 3.2 million pounds (about 5.7 million U.S. dollars given exchange rates at the time) (p. 583). So privileging RCTs in evidence hierarchies privileges certain actors over others as well. The pharmaceutical companies who can afford to implement these methods have a strong incentive to find benefits and ignore harms from their products. Making matters worse, the evidence presented in this article suggests that RCTs are not epistemically superior to other levels in the evidence hierarchy nor are they necessarily superior to other ways of knowing not mentioned in the evidence hierarchies.
EBM makes the same mistakes that Kuhn (1962) and other philosophers of science make — they overlook the very real problem of corporate influence. Gupta (2003) writes:
EBM is uncritical in that it does not build any strategies into its critical appraisal scheme to scrutinize the potentially biasing effects of the source of funding nor does it equip clinicians with the tools to assess their impact. Furthermore, it is permissive of source-of-funding bias. In its relentless pursuit of an ever-greater quantity of evidence, EBM does not acknowledge how the interests of the private research funders (such as pharmaceutical companies) might differ or even directly conflict with the interests of clinicians and patients. EBM thereby creates and fosters the illusion that social processes that contribute to EBM and social consequences of EBM are non-existent, or at least, irrelevant.
Jadad and Enkin (2007) argue that sources of bias are potentially limitless and they identify sixty of the most common types. So simply controlling for selection bias is not sufficient to guarantee scientific integrity. Furthermore, it is not even clear that RCTs as currently practiced actually prevent selection bias:
A research group conducted a systematic review of 107 RCTs about a particular medical intervention, using three popular QATs [Quality Assessment Tools] (Hartling et al. 2011). This group found that allocation concealment was unclear in 85% of these RCTs, and that the vast majority of the RCTs were at high risk of bias. Another group randomly selected eleven meta-analyses involving 127 RCTs on medical interventions in various health domains (Moher et al. 1998). This group assessed the quality of the 127 RCTs using QATs, and found the overall quality to be low: only 15% reported the method of randomization, and even fewer showed that subject allocation was concealed (Stegenga, 2015).
Perhaps the authors of these studies were simply careless in describing their methods. But given that directors of Contract Research Organizations boast of their ability to deliver the results desired by their clients (Petryna, 2007 in Mirowski, 2011) it seems reasonable to wonder whether double-blind randomization is actually happening at all in some clinical trials that purport to be RCTs.
6. Case reports and observational studies are often just as accurate as RCTs.
The definition of a case report in the Dictionary of Epidemiology is notable for its internal contradiction:
Case reports: Detailed descriptions of a few patients or clinical cases (frequently, just one sick person) with an unusual disease or complication, uncommon combinations of diseases, an unusual or misleading semiology, cause, or outcome (maybe a surprising recovery). They often are preliminary observations that are later refuted…. They may also raise a thoughtful suspicion of a new adverse drug event and are an important means of surveillance for rare clinical events. They help to reflect on and learn from medical error (citing Fletcher et al., 2014; Haynes et al., 2006; Koepsell and Weiss, 2003, Vandenbroucke, 2001; Pollock, 2012, and Sackett et al. 1991 in Porta, 2014).
So on the one hand, it is held that case reports are often refuted (even though no reference is supplied) and on the other hand, case reports “may also raise a thoughtful suspicion” (Porta, 2014).
Case reports are second from the bottom in the CEBM evidence hierarchy, ranked above only “expert opinion” and below the threshold that many epidemiologists consider worth reading. “First reports” are case reports of the first recorded incidence of a new disease or adverse event in reaction to a new drug (or new use of an existing drug). But what is the actual evidence as to the reliability of such reports?
Venning (1982) examined 52 first reports of suspected adverse drug reactions published in BMJ, the Lancet, JAMA, and NEJM in 1963. He followed up on each of these reports 18 years later to assess whether in fact they had subsequently been verified.
-
“Of 52 first reports, five were deliberate investigations into potential or predictable reactions, and in each case causality was reasonably established.”
-
The remaining 47 were what Venning calls “anecdotal” which is never defined but the context in the rest of the article suggests nine or fewer cases, and often just one case, with no control group. Venning found that “35 out of 47 anecdotal reports were clearly correct and that some of the remaining 12 unverified reports may also have represented true adverse reactions…” So 75% of these anecdotal reports were later confirmed to be correct and there was no proof of any false positives.
-
The remaining 12 adverse reactions were associated with syndromes that were either so rare, that there were not many other cases to compare them with or so common that it was difficult to separate out the effect of the drug versus chance (Venning, 1982).
When one compares the 75% success rate of anecdotal first reports with the fact that 75-80% of the most widely cited cancer RCTs cannot be replicated (Prinz, Schlange, and Asadullah, 2011; Begley and Ellis, 2012), the decision to place RCTs at the top of the CEBM evidence hierarchy, while denigrating case series, appears unwarranted.
Three studies from the early 2000s confirm that RCTs are not superior to observational studies.
-
Benson and Hartz (2000) found “little evidence that estimates of treatment effects in observational studies reported after 1984 are either consistently larger than or qualitatively different from those obtained in randomized, controlled trials.”
-
Concato, Shah, and Horwitz (2000) write, “[t]he results of well-designed observational studies… do not systematically overestimate the magnitude of the effects of treatment as compared with those in RCTs on the same topic.”
-
Petticrew and Roberts (2003) maintain that the particular research question should be matched with the appropriate research methodology in a matrix rather than a hierarchy. Furthermore, they argue, “in certain circumstances the hierarchy may even be inverted, placing for example qualitative research methods on the top rung.”
In 2017, Thomas Frieden, the former Director of the CDC, made the case in the New England Journal of Medicine that a wide range of different study types can have a positive impact on patients and policy. He makes the simple point that each type of study has strengths and weaknesses and the study type should match the type of problem the researchers are trying to address. He points out that alternative data sources are “sometimes superior” to RCTs.
So a wide range of different types of evidence can be valid and help inform clinical decision-making and yet the current practice of EBM systematically excludes everything other than the large RCTs favored by pharmaceutical companies.
7. EBM is not based on evidence that it improves health outcomes.
Numerous authors, including Sackett and his colleagues, have acknowledged that EBM violates its own evidence-based norms because “there is no evidence that EBM is a more effective means of pursuing health than medicine-as-usual” (Norman 1999 in Gupta, 2003).
Upshur (2003) notes that, “Ironically, the creation of these classifications has not as yet been informed by research but is driven in large part by expert opinion.” Defenders of EBM (such as Reilly, 2004) state that such evidence is not provided because it “cannot be proved empirically.” Yet that is not exactly true. One could easily create a natural experiment that compares patient outcomes between two equally ranked hospitals where one continues with business as usual and another implements EBM. While not exactly an RCT, there would be ways to compare before and after results within and between hospitals and even blind investigators.
Upshur and Tracy (2004) write:
[T]he entire edifice of evidence hierarchies is not based on systematic research at all, but on expert judgment or consensus. In other words, the warrant or justification for viewing evidence in such a hierarchical structure rests on the lowest form of evidence, that is, the beliefs of a few (p. 200). Also, the benefit that evidence-based approaches bring to patients is as unproven as the evidence hierarchy itself.
EBM began with the assumption that surely it would improve patient outcomes but there is little evidence to support that assumption.
A fundamental assumption of EBM is that practitioners whose practice is based on an understanding of evidence from applied health care research will provide superior patient care compared with practitioners who rely on [an] understanding of basic mechanisms and their own clinical experience. So far, no convincing direct evidence exists that shows that this assumption is correct (Haynes, 2002, in Upshur, 2005).
It’s interesting to note that the rise in chronic illness in the United States (1986 to the present) roughly corresponds to the rise in EBM in the medical profession (1992 to the present). EBM has been completely unable to stop the rise in chronic illness (particularly among children) but the rise in the stock market value of pharmaceutical companies since 1992 has been spectacular.
8. EBM and evidence hierarchies reflect authoritarian tendencies in medicine.
A number of authors have highlighted the authoritarian tendencies of EBM.
Shahar (1997) was one of the earliest critics to note the authoritarian tendencies of the EBM movement:
I think that, mistakenly, they [supporters of EBM] call for a new type of authoritarianism, hidden behind an amorphous entity called evidence-based medicine. They suggest replacing healthy scientific debates, in which no one should claim authority over the truth, with authorities of scientific knowledge — readers of the literature who will announce a verdict about the evidence and ensure that their verdict is properly executed.
Rosenfeld (2004) is fulsome in her praise for the early days of EBM:
In the 1990s, EBM streaked like a comet across the medicine skies. The advent of EBM was like the translation of the vulgate bible into English by John Wycliffe in the 15th century or the later publication by Tyndale and Coverdale in the 16th century. It was revolutionary. It was populism. It was a change. The practising doctor was going to be able to understand the evidence behind clinical practice, or the lack thereof. EBM gave us the tools to evaluate our daily practice.
But Rosenfeld (2004) then argues that those promising early days have receded to reveal a much more troubling current reality:
[D]uring the last 3 years, EBM has gone from a tool to a religious doctrine and fixed dogma. There are its priests — men and women who are known for practising and preaching EBM and changing the books and literature. You have to have one of these priests on every board and journal, or you are not up to date. Anyone who speaks against these priests is blaspheming EBM, and obviously unscientific or backward. There are thousands of acolytes, those who have heard the word and will accept nothing else.
Rosenfeld (2004) is especially critical of the EBM gatekeepers that prepare meta-analyses for consumption by the wider medical community:
There are secretive organizations that create the dogma — such as the Cochrane group, the ‘Task Force’, or Best Evidence. Who are these people? We know where they are located and sometimes their names, but we must blindly believe in their methods. They come up with conclusions that are published and then the conclusions become codified…. Only a few sources are now considered ‘true’ or reliable EBM. Some organizations list 11-15 ‘proper’ and ‘acceptable’ sources of EBM. All else, including good research, books, and reviews, are not evidenced-based, and may not be used.
Rosenfeld (2004) concludes: “We have come full circle to faith-based medicine. We are encouraged and, even, forced to mould our practice of medicine to the authority of those practitioners of EBM that are ‘approved’ and ‘acceptable.’”
Upshur (2005) similarly recounts the shift from the joyful early days of EBM to its more troubling present form:
[I]t seems a new orthodoxy is emerging, as resistant to criticism and reflection as the “paradigm” it sought to replace. The joy of inquiry, questioning, and uncovering inconsistencies and paradoxes in the belief structure of medicine has given way to what seems to be an adherence to a near-religious belief. Assertion has replaced argument. It is no coincidence that the cover of the British Medical Journal’s 10-year anniversary reflection on EBM features a picture of what looks like three priests in a high tower. Evidence is a concept health care is avidly embracing, for legitimacy and authenticity. However, it has come to be more like familiar advertising slogans, an attractive package, a branding exercise, one that draws people in with its seductive promises of being more rigorous and scientific in the application of medical principles.
9. Evidence hierarchies have reshaped the practice of medicine for the worse.
Evidence hierarchies have reshaped the practice of medicine in ways that are advantageous to pharmaceutical companies and disadvantageous to doctors and patients.
Upshur (2003), recounting a story from his medical practice, gives a glimpse of how pharmaceutical companies use EBM to sell their products:
A pharmaceutical company distributed evidence-based guidelines to my clinic. According to the guidelines, level 1 (best) evidence required one well-designed randomized control trial, and a grade A recommendation required one level 1 study. The guidelines were accompanied by a report of the randomized trial sponsored by that same company and published in a peer-reviewed journal. By disseminating the guidelines with the supportive paper, the company sought to persuade me that I would be following evidence-based guidelines if I prescribed the drug (p. 673).
The process that doctors are taught to use in connection with EBM is an idealized process. In the real world, doctors rarely have the time to follow all of the steps. So instead, they use shortcuts supplied by medical publishers and others.
Recently, there has been acknowledgement of a distinction between evidence-based practitioners and evidence users (Guyatt et al. 2000). Particularly in primary care, there is a trend toward using pre-appraised sources of evidence. One of the most popular of these sources is InfoPOEMs (patient-oriented evidence that matters), a daily email service that provides summaries of research studies relevant to primary care [now part of Essential Evidence Plus]. Each summary is accompanied by the level of evidence, using the Oxford Centre nomenclature (Upshur, 2003).
This shift from evidence-based practitioner to evidence user is presented by proponents of EBM as an acceptable alternative to the idealized process. Yet if one examines these developments in their wider context, it is clear how problematic they are. What started out as a process to empower doctors now has doctors essentially taking orders from the pharmaceutical companies who run most of the clinical trials. Even though many of these studies are not replicable, harried doctors with detailers in their office showing them the latest “evidence-based medicine” are going to feel enormous pressure to conform. Clinicians who do not follow the latest EBM guidelines may also wonder whether such independent thinking might expose them to additional risk of malpractice suits.
Groopman (2007) in How Doctors Think describes the impact of EBM on the hospital workplace and the mindset of doctors:
Each morning as rounds began, I watched the students and residents eye their algorithms and then invoke statistics from recent studies. I concluded that the next generation of doctors was being conditioned to function like a well-programmed computer that operates within a strict binary framework.
What likely started out with good intentions, can become paint-by-numbers medicine that constrains the wisdom and creativity of some of our finest minds:
Clinical algorithms can be useful for run-of-the-mill diagnosis and treatment… But they quickly fall apart when a doctor needs to think outside their boxes, when symptoms are vague, or multiple and confusing, or when test results are inexact. In such cases — the kinds of cases where we most need a discerning doctor — algorithms discourage physicians from thinking independently and creatively. Instead of expanding a doctor’s thinking, they can constrain it (Groopman, 2007).
Goldenberg (2009) provides an extraordinary account of the political economy of EBM and how EBM shapes the mode of production in medicine:
Given the demands of keeping up with the literature, the time associated with evaluating the abundance of clinical research, and the importance of “getting it right,” it did not take long for EBM to replace its earlier call for individual critical appraisal of the evidence by practicing clinicians with a veritable industry of systematic review and meta-analysis (available for a fee, typically through electronic databases). While thought by many to be timely and useful, the availability of meta-analyses and clinical summaries immediately derails EBM’s early anti-authoritarian programmatic. The initial program of equipping all practicing physicians with critical appraisal skills (and “a computer at every bedside”) was intended to democratize medicine by discarding the hierarchical nature of expert opinion and received wisdom. That very authoritarianism seems to be restored by the creation of “expert” EBM sources that proliferate clinical guidelines, meta-analyses, educational products, electronic decision support systems, and all things worthy of the brand name “evidence-based medicine” to a captive and paying audience of clinicians who desire to be “evidence-based practitioners.”
EBM is now a brand and everything that goes along with being a brand — a shortcut to decision making, very powerful at shaping decisions, essential to marketing and profit but not a very precise indicator of the quality of the contents.
10. Evidence hierarchies objectify and/or overlook patients.
EBM objectifies patients in ways that run counter to the traditional practice of medicine and more recent paradigms such as “patient centered medicine.” Upshur and Tracy (2004), write, “[I]t is interesting to note that patients do not become relevant until Step 4 [in the EBM process outlined by Sackett et al., 1997, summarized above]. In fact, patients are seen as passive objects that have evidence applied to them after the information has been extracted from them.”
Such discounting of patients’ experiences and inherent subjectivity would seem to be a violation of fundamental values in medicine and yet it is the dominant philosophy of medicine today. Upshur (2005) writes:
Patients are seen very much as objects from which information is to be gleaned and then inspected. Nowhere in the EBM process is listening to patients and their concerns, and legitimizing their questions, regarded as important. Gadamer (1975) writes of the hermeneutical priority of the question and how this establishes direction and dialogical relationship. Which question is considered or reflected upon establishes whether the relationship between doctor and patient is one of inspectorial power or of dialogue, mutual respect, and deliberation. In the sense that the voice of the patient is explicitly excluded in the steps of EBM, except insofar as it is a voice of pathological information that can be transformed into searchable terms, it is no wonder that proponents of EBM can still write of the problematic nature of including patient values and perspectives (see, for example, Haynes 2002). They are omitted from the process by definition.
I will return to this issue below in my discussion of implications for the autism epidemic.
V. The AMA’s 2002, 2008, and 2015 evidence hierarchies
In 2002, the American Medical Association created its own evidence hierarchy, The Users’ Guide to the Medical Literature (Guyatt and Rennie 2002) and it contained a fascinating twist. It resembled the CEBM, except at the very top of the hierarchy, the AMA listed N-of-1 randomized control trials.
Table 2
Source: Guyatt and Rennie (2002), p. 7.
An N-of-1 trial is a clinical trial in which a single patient is the entire sample population. N-of-1 trials can be double-blinded (both patient and doctor do not know the treatment vs. the placebo) and the order of treatment and control can be randomized using various patterns (Guyatt, et al., 1986, p. 889-890).
N-of-1 medicine is an important step in the right direction because it reflects a philosophy of medicine that is in keeping with the heterogeneity of the human population. But few formal N-of-1 trials are conducted each year. By 2008, Kravitz et al. wrote, “What ever happened to N-of-1 trials?” noting that “Despite early enthusiasm, by the turn of the twenty-first century, few academic centers were conducting n-of-1 trials on a regular basis” (p. 533). Lillie et al. (2011) write, “Despite their obvious appeal and wide use in educational settings, N-of-1 trials have been used sparingly in medical and general clinical settings” (p. 161).
Curious about the dearth of N-of-1 trials, I started researching what happened. And what I discovered shocked me.
In 2000, the GRADE (Grading of Recommendations Assessment, Development and Evaluation) Work Group began to meet. Gordon Guyatt was one of its leaders. By 2004 they published their framework and it is the opposite of transparent — it takes the different levels from the evidence hierarchy and converts them into a “quality scale” — “high, moderate, low, and very low.” At the top of their evidence hierarchy is RCTs. So according to GRADE, if a study is an RCT, it is considered “high quality” which is defined as “We are very confident that the true effect lies close to that of the estimate of the effect. Further research is very unlikely to change our confidence in the estimate.”
GRADE converted a system based on data to one based on normative labels — “high quality,” “high confidence,” even though as I have shown above, RCTs are not more reliable than other forms of evidence. GRADE is an opaque wrapper that hides what’s inside the model and gives all the power in decision-making to the people preparing the recommendations. Governments and public health agencies including the WHO, FDA, and CDC love GRADE because it tells people what to do in no uncertain terms without having to deal with the messiness of odds ratios, confidence intervals, and p values.
In 2008, the American Medical Association published a new edition of The Users’ Guide to the Medical Literature and N-of-1 trials had been downgraded below systematic reviews of RCTs. Given how these evidence hierarchies work, anything below the first tier is considered inferior and ignored which means that the AMA had abandoned N-of-1 as a valid methodology for clinical decision-making.
The third edition of The Users’ Guide to the Medical Literature published in 2015 fully embraces GRADE as the AMA’s preferred framework for making prevention and treatment decisions.
I saw GRADE in use when I watched every meeting the FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) and the CDC’s Advisory Committee on Immunization Practices (ACIP) in 2022 and 2023. GRADE is a tool to give legitimacy to ANY medical intervention no matter how abysmal the data. For example, the FDA and CDC used GRADE to authorize:
-
The use of the Pfizer COVID vaccine in adults even though more people died in the treatment than the control group;
-
The use of Covid vaccines in children even though the clinical trial showed no clinically significant benefit to children; and
-
Covid vaccine boosters for all age groups with no human testing whatsoever and only 28 days of antibody results in six mice.
So within 13 years (from the first edition in 2002 to the third edition in 2015) the AMA went from the best-in-class evidence hierarchy that acknowledged individual difference to a cartoonish monstrosity, GRADE, that is just a tool for laundering bad data on behalf of the pharmaceutical industry. In the process the AMA sold out the doctors in their association and the patients in their care to the drug makers.
VI. More details on the corporate takeover of EBM
Ioannidis (2016) recounts his conversations and correspondence with David Sackett over the course of many years about how EBM changed since its initial conception:
As EBM became more influential, it was also hijacked to serve agendas different from what it originally aimed for. Influential randomized trials are largely done by and for the benefit of the industry. Meta-analyses and guidelines have become a factory, mostly also serving vested interests. National and federal research funds are funneled almost exclusively to research with little relevance to health outcomes. We have supported the growth of principal investigators who excel primarily as managers absorbing more money…. Under market pressure, clinical medicine has been transformed to finance-based medicine (Ioannidis, 2016, p. 82).
One of the many problems with EBM is that focusing on poorly defined notions of “quality” sometimes overlooks important dynamics and variables.
Now that EBM and its major tools, randomized trials and meta-analyses, have become highly respected, the EBM movement has been hijacked. Even its proponents suspect that something is wrong (Greenhalgh et al. 2014 and Greenhalgh, 2012). The industry runs a large share of the most influential randomized trials. They do them very well, they score better on “quality” checklists (Khan et al. 2012), and they are more prompt than non-industry trials to post or publish results (Anderson et al. 2015). It is just that they often ask the wrong questions with the wrong short-term surrogate outcomes, the wrong analyses, the wrong criteria for success (e.g., large margins for noninferiority), and the wrong inferences (Every-Palmer and Howick, 2014; Turner et al. 2008; and Lexchin et al. 2003)… The industry is also sponsoring a large number of meta-analyses currently (Ebrahim et al. 2016). Again, they get their desirable conclusions (Jørgensen et al. 2006) (in Ioannidis, 2016).
As I pointed out in chapter 5 of my doctoral thesis, even meta-analyses and systematic reviews, which sit at the top of most evidence hierarchies, are contaminated by corporate influence. Iaonnidis (2016) notes that even the widely respected Cochrane Collaboration “may cause harm by giving credibility to biased studies of vested interests through otherwise respected systematic reviews” (p. 84).
Ioannidis (2016) provides a vivid illustration of the current mode of production in medicine and how EBM has become the corporate tail wagging the dog.
In most developed countries, clinicians are under tremendous market pressure. Most discussions in department meetings are about money. One can sense the pressure to deliver services, to capture the largest possible market share (a synonym for ‘‘patients’’), to satisfy customers (synonyms for ‘‘humans’’), to get high satisfaction scores, to charge more, to perform more procedures, and to tick off more items on charge forms. (As an aside, a nice joke is that these charge driven electronic health records are then used for research.) This is not what I thought medicine would be about, let alone EBM. This is mostly finance-based medicine. I would not blame anyone. These physicians have no other option. This is how the world works; they are fighting to keep their jobs. Yet, how likely is it that physicians will design studies whose results may threaten their jobs by suggesting that less procedures, testing, interventions are needed? How likely is it that, if they do design such studies, they will accept results suggesting that they should quit their jobs?… Is EBM doomed to be heartily accepted only when it leads to more medicine, even if this means less health (Glasziou et al. 2013; Grady and Redberg, 2010)?… In some settings, we are close or past the tipping point where medicine diminishes rather than improves well-being in our society (p. 85).
This is a startling turn of events. Doctors are often seen as heroic, selfless, and wise. EBM was conceived with the best of intentions to further improve medical practice. And yet, Ioannidis (2016) is openly stating that the whole endeavor has been hijacked to serve corporate ends rather than patient needs.
VII. Analysis and implications for the autism epidemic
I want to highlight nine facets of EBM and evidence hierarchies as they apply to the autism epidemic.
1. CEBM, GRADE, and other evidence hierarchies replace the varied ways of knowing with a single tool — RCTs. Supporters of EBM seem to base their model entirely on an idealized view of science. A more “evidence-based” approach would be to read the CEBM evidence hierarchy in the context of how science is actually done. Most RCTs are done at overseas (usually Chinese) CROs (Mirowski, 2011). 50% (Horton, 2015) to 80% (Prinz, Schlange, and Asadullah, 2011; Begley and Ellis, 2012) of what is published is not replicable. To claim that RCTs are the “highest quality” evidence and that one should not bother to read anything else is clearly untenable, unscientific, and not in the interests of patients.
2. It is striking how much the CEBM evidence hierarchy, GRADE, and other evidence hierarchies degrade the contribution of doctors. Starr (1982, 1997) and others have pointed out that doctors have been gradually losing agency as capital and corporations have come to play an ever-greater role in medicine. But to place a doctor’s “expert opinion” at the bottom of the hierarchy, below even “poor quality cohort and case-control studies” is an example of epidemiologists putting their own work above those actually practicing and interfacing with patients in the real world. Instead of viewing doctors as trusted advisors whose instincts, experience, and intuition are key to successful outcomes, the CEBM, GRADE, and other evidence hierarchies regard doctors as the least reliable form of evidence. In the process, the role of the doctor shrinks from discernment to obedience.
3. Individual patients are nowhere to be found in the CEBM evidence hierarchy, GRADE, or other evidence hierarchies. One’s own perspective and insights into one’s disease state do not even make it onto the chart at all. The experiences and insights of patients, the views of doctors, and alternative forms of evidence can provide the data that challenge paradigms. To denigrate these ways of knowing leaves existing paradigms in place even when they have failed to serve the public.
4. EBM has changed the practice of medicine. “In 2023, the United States had 1,010,892 active physicians of which 851,282 were direct patient care physicians” (Association of American Medical Colleges, 2024). There are multiple ways of knowing including RCTs, meta-analyses and systematic reviews, prospective and retrospective cohort studies, case-control studies, cross-sectional studies, ecological studies, observational studies, case reports and series, registries, bench research, and more. In a crisis like autism, it would seem that all available resources — the talents of over a million trained professionals and multiple ways of knowing would be brought to bear on stopping the epidemic. By contrast, EBM represents a deskilling and circumscribing of the practice of doctors, an exclusion of multiple streams of evidence, and a turning over of the process of discovery to a smaller number of specialists, often in the employ of pharmaceutical companies. The result is a calcified practice of medicine, ill-equipped to respond to the crises it faces and the crises to which it contributes.
5. From the corporate-funded studies that produce the outcomes desired by their patrons, to the studies that never get funded, to the studies that get funded only to get quashed, to the studies that get completed but that never lead to regulation, to the rules of “scientific” evidence in the courts that protect corporations and harm plaintiffs, to the philosophy of medicine that discounts methods for detecting harms and favors corporate ways of knowing over other valid epistemologies — medicine in the U.S. is a system that is more hegemonic than scientific; more an expression of power relations than a method for producing good data or improved health outcomes for patients. It is a system that is quite good at protecting the profitable status quo but not very good at producing the sort of open-ended inquiry that can lead to the paradigm shifts necessary to stop the autism epidemic.
6. Given a philosophy of medicine that privileges a certain sort of epidemiology to the exclusion of all other forms of knowing, is it any wonder then that doctors routinely dismiss the thousands of parents who try to explain to their doctors the origins of their child’s autism symptoms (Campbell, 2010; Habakus and Holland, 2011; Handley, 2018)? The experiences of these parents were dismissed long before the family ever walked in the door — they were excluded in medical school when the future doctor was studying evidence-based medicine and learning to follow an epistemology that favors corporate interests and excludes other ways of knowing.
7. It is beyond infuriating that evidence-based medicine has spent more than three decades extolling the virtues of double-blind, randomized, controlled trials, and yet all of the so-called RCTs in connection with vaccines are fraudulent. Everyone knows that they are fraudulent (even though the mainstream medical profession tries to excuse this fraud). In clinical trials for vaccines, the control group is not given an inert saline placebo and is given another toxic vaccine or the toxic adjuvants from the trial vaccine instead. The Informed Consent Action Network (2023) has the receipts. So at the end of the day, the entire evidence-based medical system — including the tens of thousands of published papers and the thousands of careers dedicated to promoting EBM — is a giant theatrical production to empower epidemiologists and enrich the pharmaceutical industry. The professionals involved do not believe their own stated values and are actively participating in the mass poisoning of the population and the destruction of civilization. This is one of the most extreme examples of a failure of moral courage and dereliction of scientific duty in the history of the world.
8. If one wants to be scientific, it would follow that one should turn to those who are making important discoveries first. The parents’ group, the National Society for Autistic Children (founded by Bernard Rimland, now the Autism Society of America) proposed an environmental influence on autism in 1974 (Olmsted and Blaxill, 2010), forty-two years before Project TENDR reached the same conclusion (Bennett et al., 2016). By the mid 1990s, it was common knowledge among parents of children with autism, that autism had a gastrointestinal component (Kirby, 2005) — two decades before the microbiome became the “new frontier in autism research” (Mulle, Sharp, and Cubells, 2013). We know that EBM is a fraud because it ranks rigged corporate studies ahead of the paradigm-shifting breakthroughs discovered by parents that are actually helping autistic children.
9. Going forward, any system of medicine in connection with autism must start with the individual child and his/her family as the highest form of evidence (because obviously they are). All forms of data, no matter how unconventional or “outside the box,” must be brought to bear on supporting recovery and preventing this injury from happening to others. Rigged corporate RCTs have no place in actual medicine; their only appropriate use is as evidence of crimes against humanity in future Nuremberg trials of pharmaceutical executives and their enablers in government. The revolution we seek is thus a return to actual science instead of the genocidal corporate nonsense posing as evidence-based medicine today.
REFERENCES
Association of American Medical Colleges (2024). “U.S. Physician Workforce Data Dashboard.” https://www.aamc.org/data-reports/data/2024-key-findings-and-definitions
Begley, C. G., & Ellis, L. M. (2012, March 28). Raise standards for preclinical cancer research. Nature, 483(7391), 531–533. https://doi.org/10.1038/483531a
Bennett D, et al. (2016). Project TENDR: Targeting Environmental Neuro-Developmental Risks. The TENDR Consensus Statement. Environmental Health Perspectives, 124, A118-A122. http://doi.org/10.1289/EHP358
Benson, K. & Hartz, A. J. (2000, June). A comparison of observational studies and randomized, controlled trials. NEJM;342(25):1878–1886. https://doi.org/10.1056/nejm200006223422506
Berwick, D. M. (2005). Broadening the view of evidence-based medicine. Quality and Safety Health Care, 14, 315-316. http://doi.org/10.1136/qshc.2005.015669
Borgerson, K. (2009). Valuing Evidence: Bias and the Evidence Hierarchy of Evidence-Based Medicine. Perspectives in Biology and Medicine, 52(2), 218-233. http://doi.org/10.1353/pbm.0.0086
Campbell, J. (2010). Parents Voice: Children’s Adverse Outcomes Following Vaccination. http://www.followingvaccinations.com/
Concato, J., Shah, N., & Horwitz, R. I. (2000, June 22). Randomized, controlled trials, observational studies, and the hierarchy of research designs. NEJM;342(25):1887–1892. https://doi.org/10.1056/nejm200006223422507
Daly, J. (2005). Evidence-Based Medicine and the Search for a Science of Clinical Care. Berkeley: University of California Press.
Eddy, D. M. (1990). Practice Policies: Guidelines for Methods. JAMA, 263(13), 1839-1841. http://doi.org/10.1001/jama.1990.03440130133041
epidemiology. (n.d.). Merriam-Webster.com. https://www.merriam-webster.com/dictionary/epidemiology
Evidence-Based Medicine Working Group, (1992, November 4). Evidence-Based Medicine: A New Approach to Teaching the Practice of Medicine. JAMA, 268(17), 2420-2425. http://doi.org/10.1001/jama.1992.03490170092032
Feinstein, A. R. (1967). Clinical Judgment: The Theory and Practice of Medical Decision. New York, NY.
Fletcher, R. H., Fletcher, S. W., & Wagner, E. H. (1982). Clinical epidemiology: The essentials. Baltimore, MD: Williams & Wilkins.
Frieden, T. R. (2017, August 3). Evidence for Health Decision Making — Beyond Randomized, Controlled Trials. NEJM; 377:465-475. https://www.nejm.org/doi/full/10.1056/NEJMra1614394
Gadamer, Hans-Georg. (1975). Truth and Method. New York: Seabury Press.
Goldenberg, M. J. (2009, Spring). Iconoclast or Creed?: Objectivism, Pragmatism, and the Hierarchy of Evidence. Perspectives in Biology and Medicine, 52(2). http://doi.org/10.1353/pbm.0.0080
Groopman, J. (2007). How Doctors Think. Boston: Houghton Mifflin Company.
Gupta, M. (2003). A critical appraisal of evidence-based medicine: Some ethical considerations. Journal of Evaluation in Clinical Practice, 9(2), 111–121. https://doi.org/10.1046/j.1365-2753.2003.00382.x
Guyatt, G., Sackett, D., Taylor, D. W., Ghong, J., Roberts, R., & Pugsley, S. (1986). Determining optimal therapy—randomized trials in individual patients. New England Journal of Medicine, 314(14), 889-892. https://www.nejm.org/doi/10.1056/NEJM198604033141406
Guyatt, G. H., & Rennie, D. (Eds.). (2002). Users’ guides to the medical literature: A manual for evidence-based clinical practice. American Medical Association.
Guyatt, G. H., Rennie, D., Meade, M. O., & Cook, D. J. (Eds.). (2008). Users’ guides to the medical literature: A manual for evidence-based clinical practice (2nd ed.). McGraw-Hill.
Guyatt, G. H., Rennie, D., Meade, M. O., & Cook, D. J. (Eds.). (2015). Users’ guides to the medical literature: A manual for evidence-based clinical practice (3rd ed.). McGraw-Hill Education.
Habakus, L. K., and Holland, M. (Editors). (2011). Vaccine Epidemic. New York: Skyhorse Publishing.
Handley, J. B. (2018). How to end the autism epidemic. Chelsea Green Publishing.
Hanemaayer, A. J (2016, December). Evidence-Based Medicine: A Genealogy of the Dominant Science of Medical Education. Journal of Medical Humanities, 37(4), 449-473. http://doi.org/10.1007/s10912-016-9398-0
Haynes, R. 2002. What kind of evidence is it that evidence-based medicine advocates want health care providers and consumers to pay attention to? BMC Health Services Research 2:3. https://doi.org/10.1186/1472-6963-2-3
Hazell, L., & Shakir, S. A. W. (2006). Under Reporting of Adverse Drug Reactions: A Systematic Review. Drug Safety, 29(5), 385–396. https://link.springer.com/article/10.2165/00002018-200629050-00003
Horton, R. (2015). Offline: What is medicine’s 5 sigma? The Lancet, 385(9976), 1380. https://doi.org/10.1016/S0140-6736(15)60696-1
Howick, J. H. (2011). The Philosophy of Evidence-based Medicine. Oxford: Wiley-Blackwell.
Informed Consent Action Network (2023, October 18). Childhood Vaccine Trials Summary Chart. https://icandecide.org/article/childhood-vaccine-trials-summary-chart/
Ioannidis, J. P. A. (2016). Evidence-based medicine has been hijacked: a report to David Sackett. Journal of Clinical Epidemiology, 73, 82-86. http://doi.org/10.1016/j.jclinepi.2016.02.012
Jadad, A. R. and Enkin, M. W. (2007). Randomised Controlled Trials: Questions, Answers and Musings, 2nd Edition. Malden, Massachusetts: BMJ Books.
Kesselheim, A. S., Mello, M. M., & Studdert, D. M. (2011). Strategies and practices in off-label marketing of pharmaceuticals: a retrospective analysis of whistleblower complaints. PLoS Med, 8(4), http://doi.org/10.1371/journal.pmed.1000431
Kirby, D. (2005). Evidence of Harm: Mercury In Vaccines and the Autism Epidemic: A Medical Controversy. New York: St. Martin’s Press.
Kravitz, R. L., Duan, N., Niedzinski, E. J., Hay, M. C., Subramanian, S. K., & Weisner, T. S. (2008). What Ever Happened to N-of-1 Trials? Insiders’ Perspectives and a Look to the Future. The Milbank Quarterly, 86(4), 533–555. http://doi.org/10.1111/j.1468-0009.2008.00533.x
Lillie, E. O., Patay, B., Diamant, J., Issell, B., Topol, E. J., & Schork, N. J. (2011). The n-of-1 clinical trial: the ultimate strategy for individualizing medicine? Personalized Medicine, 8(2), 161-173. http://doi.org/10.2217/pme.11.7
Medicines and Healthcare Products Regulatory Agency. (2014, 3 November). Professor Sir Michael Rawlins appointed Chair of Medicines and Healthcare Products Regulatory Agency. Press release. https://www.gov.uk/government/news/professor-sir-michael-rawlins-appointed-chair-of-medicines-and-healthcare-products-regulatory-agency
Mirowski, P. (2011). Science-Mart: Privatizing American Science. Harvard University Press.
Mulle, J. G., Sharp, W. G., and Cubells, J. F. (2013). The Gut Microbiome: A New Frontier in Autism Research. Current Psychiatry Reports, 15(2), 337. http://doi.org/10.1007/s11920-012-0337-0
Olmsted, D. and Blaxill, M. (2010). The Age of Autism: Mercury, Medicine, and a Man-Made Epidemic. New York: St. Martin’s Press.
Petticrew, M. & Roberts, H. (2003). Evidence, hierarchies, and typologies: horses for courses. Journal of Epidemiology & Community Health. 2003 Jul;57(7):527–529. https://doi.org/10.1136/jech.57.7.527
Porta, M. (2014). Dictionary of Epidemiology, 6th edition. Oxford: Oxford University Press.
Prinz, F., Schlange, T., & Asadullah, K. (2011). Believe it or not: how much can we rely on published data on potential drug targets? Nature Reviews Drug Discovery, 10, 712. https://doi.org/10.1038/nrd3439-c1
Rawlins, M. (2008, December). De Testimonio: on the evidence for decisions about the use of therapeutic interventions. Clinical Medicine, 8(6). http://doi.org/10.7861/clinmedicine.8-6-579
Reilly, B. M. (2004). The essence of EBM. BMJ, 329(7473), 991-992. https://pmc.ncbi.nlm.nih.gov/articles/PMC524538
Rosenfeld, J. A. (2004), The view of evidence-based medicine from the trenches: liberating or authoritarian? Journal of Evaluation in Clinical Practice, 10, 153-155. http://doi.org/10.1111/j.1365-2753.2003.00472.x
Sackett, D. L., Richardson, W. S., Rosenberg, W. M. C., and Haynes, R. B. (1997). Evidence-based medicine: how to practice and teach EBM. London: Churchill Livingstone.
Shahar, E. (1997). A Popperian perspective of the term ‘evidence-based medicine’. Journal of Evaluation in Clinical Practice, 3, 109-116. http://doi.org/10.1046/j.1365-2753.1997.00092.x
Starr, P. (1982, 1997). The Social Transformation of American Medicine. New York: Basic Books.
Stegenga, J. (2011). Is Meta-Analysis the Platinum Standard of Evidence? Studies in History and Philosophy of Science, 42, 497-507. https://doi.org/10.1016/j.shpsc.2011.07.003
Stegenga, J. (2014, October). Down the with Hierarchies. Topoi, 33(2), 313-322. http://doi.org/10.1007/s11245-013-9189-4
Stegenga, J. (2015). Herding QATs: Quality Assessment Tools for Evidence in Medicine. In. Huneman, P. et al. (eds.), Classification, Disease, and Evidence, History Philosophy and Theory of the Life Sciences 7. http://doi.org/10.1007/978-94-017-8887-8
Stegenga, J. (2016). Hollow Hunt for Harms. Perspectives on Science, 24(5), 481-504. http://doi.org/10.1162/POSC_a_00220
Straus, S. E., Glasziou, P., Richardson, W. S. and Haynes, R. B. (2005). Evidence-Based Medicine: How to Practice and Teach It. London: Churchill Livingstone.
Sur, R. L., & Dahm, P. (2011). History of evidence-based medicine. Indian Journal of Urology : IJU : Journal of the Urological Society of India, 27(4), 487-489. http://doi.org/10.4103/0970-1591.91438
Upshur, R. E. G. (2003, September 30). Are all evidence-based practices alike? Problems in the ranking of evidence. Canadian Medical Association Journal, 169(70), 672-673. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC202284/
Upshur, R. E. G. (2005, Autumn). Looking for rules in a world of exceptions: reflections on evidence-based practice. Perspectives in Biology and Medicine, 48(4), 477-489. http://doi.org/10.1353/pbm.2005.0098
Upshur, R. E. G. and Tracy, C. S. (2004, Fall). Legitimacy, Authority, and Hierarchy: Critical Challenges for Evidence-Based Medicine. Brief Treatment and Crisis Intervention, 4(3), 197-204. http://doi.org/10.1093/brief-treatment/mhh018
Venning, G. R. (1982, 23 January). Validity of anecdotal reports of suspect adverse drug reactions: the problem of false alarms. BMJ, 284(6311), 249-252. https://pmc.ncbi.nlm.nih.gov/articles/PMC1495801/
Blessings to the warriors. 🙌
Prayers for everyone fighting to stop the iatrogenocide. 🙏
Huzzah for everyone building the parallel society our hearts know is possible. ✊
In the comments, please let me know what’s on your mind.
As always, I welcome any corrections.
Click this link for the original source of this article.
Author: Toby Rogers
This content is courtesy of, and owned and copyrighted by, https://tobyrogers.substack.com and its author. This content is made available by use of the public RSS feed offered by the host site and is used for educational purposes only. If you are the author or represent the host site and would like this content removed now and in the future, please contact USSANews.com using the email address in the Contact page found in the website menu.