This article originally appeared on Focal Points and was republished with permission.
Guest post by Nicolas Hulscher, MPH
The missing microbe plays a critical role in healthy development — and its absence triples the risk of allergies, asthma, and eczema in early childhood.
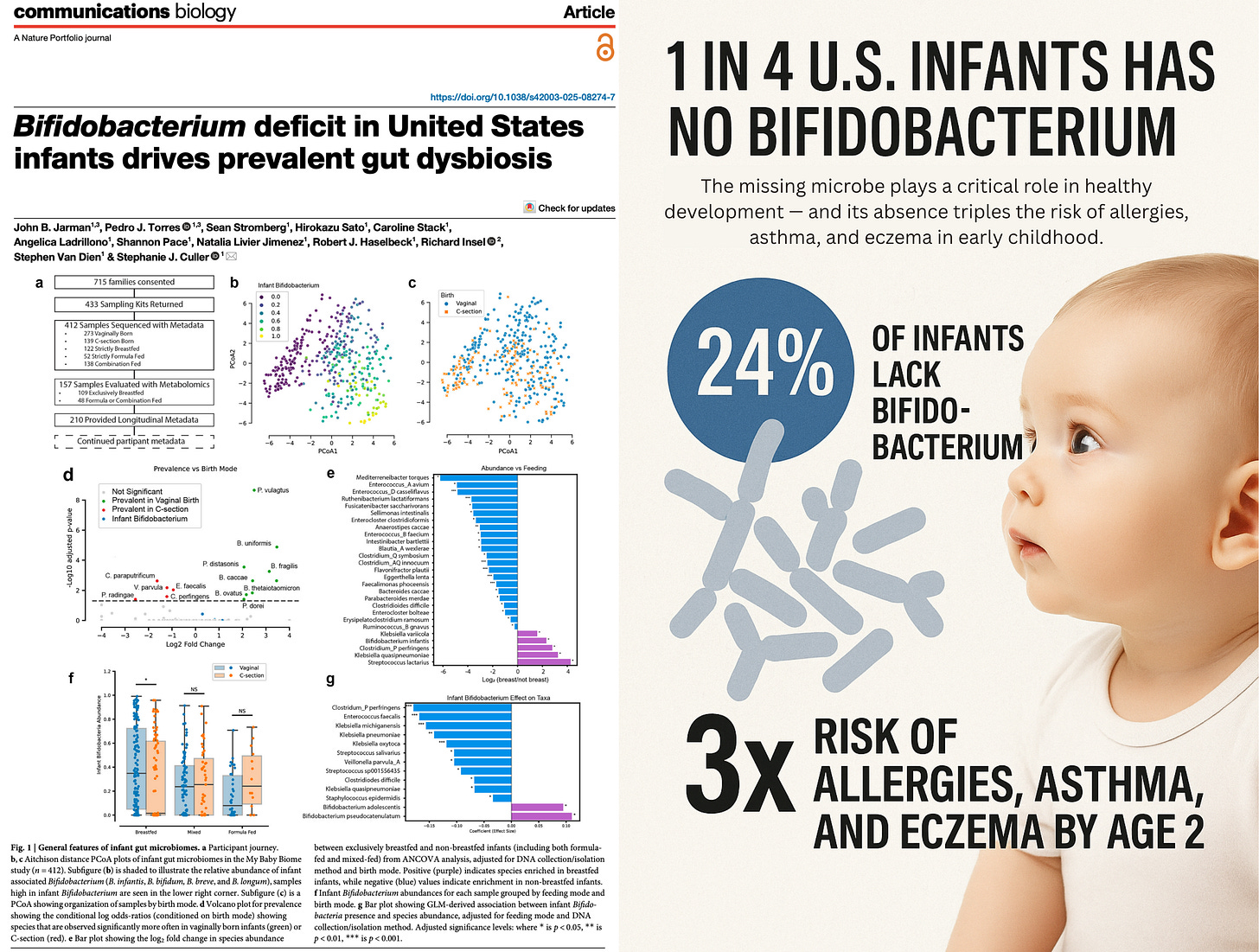
First reported by TrialSite News, a landmark new study titled “Bifidobacterium Deficit in United States Infants Drives Prevalent Gut Dysbiosis” reveals that roughly 24% of U.S. infants entirely lack detectable levels of Bifidobacterium — a cornerstone gut microbe critical for immune training and healthy development.
The My Baby Biome study is the largest nationwide investigation to date of infant gut microbiomes and metabolomes:
How the Study Was Done
Participants: 412 infants from 48 U.S. states, reflecting national racial, geographic, and birth mode diversity.
Age at sampling: 1–3 months old — a critical window before solid food introduction.
Data collected: Fecal samples, feeding/birth data, health status at 2 years.
Analyses performed:
-
Whole-genome metagenomics for microbial composition and functional capacity
-
Metabolomics to measure 79 metabolites linked to immunity and development
-
Longitudinal health surveys evaluating allergies, eczema, asthma diagnoses
Cluster analysis: Infants were grouped into 3 microbiome types (C1–C3) based on gut composition.
#ad: As someone who sits (and sometimes stands) at a desk all day, I appreciate when there’s an easy product that can help me maintain an optimal weight.
Berberine from Global Healing is a plant compound with potential effects on metabolism and blood sugar support.
Try it for yourself and see if it works for you. Use Coupon Code VFOX for 10% off.
DISCLOSURE: This is an affiliate link. I may earn a commission if you make a purchase here, at no additional cost to you.
What the Study Found
24% of U.S. infants had no detectable Bifidobacterium.
-
Even among vaginally delivered, breastfed infants, absence was common.
-
B. infantis, a crucial immune-supportive species, was detected in only 8% of infants.
Widespread immune-related disease by age 2:
-
30% of infants had been diagnosed with allergies, eczema, and/or asthma.
-
Risk was 3x higher in infants with dysbiotic microbiomes (C2 and C3 clusters).
Protective effect of B. breve:
-
Infants colonized with B. breve had a 4.8-fold lower risk of developing immune-related diseases.
-
B. longum had a weaker, non-significant protective effect.
C-section and breastfeeding can backfire together.
-
In C-section babies, breastfeeding often failed to establish Bifidobacterium — and instead enabled harmful competitors like Clostridium perfringens to colonize the gut.
Breastfeeding supported pathogens in dysbiotic guts:
-
In the absence of Bifidobacterium, breast milk oligosaccharides (HMOs) were consumed by opportunistic species — creating a pro-inflammatory metabolic profile instead of a protective one.
Pathogens hijacked HMO metabolism:
-
C. perfringens and Klebsiella pneumoniae exploited the HMO niche meant for Bifidobacterium, but lacked the full enzymatic machinery — degrading host-derived compounds inefficiently and harmfully.
Elevated antimicrobial resistance and virulence factors:
-
Infants lacking Bifidobacterium had higher levels of AMR genes and virulence factors, including lipopolysaccharide (LPS) synthesis and phage-associated genes.
-
These infants were more likely to harbor hostile microbial environments — even at just 1–3 months of age.
Disrupted metabolic signaling in dysbiotic infants:
-
Lower production of indole-3-lactate (ILA) and thiamine — both essential for immune tolerance and neurodevelopment.
-
Higher gut pH, altered bile acids, and a shift toward butyrate production (linked to Clostridium species) were also observed in dysbiotic profiles.
The industrialization of childbirth, infant nutrition, and environmental exposures appears to be erasing a keystone strain from the infant gut microbiome — Bifidobacterium, a group of beneficial bacteria critical for immune imprinting and early metabolic health. This foundational collapse leaves babies more vulnerable to immune dysfunction, allergic disease, and chronic inflammation from the start.
The introduction of synthetic mRNA injections may further exacerbate this crisis. Hazan et al found a persistent decline in Bifidobacterium abundance in all subjects 6–9 months following COVID-19 mRNA injection — with levels in each individual falling below 1% relative abundance, regardless of prior baseline:
If you want to learn more about the critical importance of the microbiome, I recommend you watch the following interview with gastroenterologist and microbiome expert Dr. Sabine Hazan:
Epidemiologist and Foundation Administrator, McCullough Foundation
www.mcculloughfnd.org
Please consider following both the McCullough Foundation and my personal account on X (formerly Twitter) for further content.
Copyright 2025 Focal Points
Click this link for the original source of this article.
Author: The Vigilant Fox
This content is courtesy of, and owned and copyrighted by, https://vigilantfox.substack.com and its author. This content is made available by use of the public RSS feed offered by the host site and is used for educational purposes only. If you are the author or represent the host site and would like this content removed now and in the future, please contact USSANews.com using the email address in the Contact page found in the website menu.