I was recently interviewed on June 3, 2025, by Nicolas Hulscher of Focal Points. We discussed the lack of a boxed warning (“black box”) for mRNA covid vaccine labeling for myocarditis–despite the peer-reviewed and published evidence of fatal outcomes. We also discussed the recent change in recommendation of the vaccines for healthy children. https://www.thefocalpoints.com/p/why-is-the-fda-still-covering-up?publication_id=1119676&post_id=165190133&r=q9str&triedRedirect=true
A boxed warning “is ordinarily used” when:
- There is an adverse reaction so serious in proportion to the potential benefit from the drug
OR
- There is a serious adverse reaction that can be prevented or reduced in frequency or severity
https://www.fda.gov/media/71866/download
In the interview, we also discussed the “New Safety Information,” sent to the manufacturers on April 17, 2025, to update the labeling:

The U.S. Food and Drug Administration (FDA) required the manufacturers to respond “within 30 calendar days of the date of this letter [sent on April 17]…”
I shared my concerns during the interview regarding this labeling change:

-and-

On June 25, 2025, the FDA approved the draft content of the “new safety information,” submitted, in the case of Pfizer, on June 18, 2025. https://www.fda.gov/media/187265/download?attachment
In summary:
On April 17, 2025: FDA initially requested Pfizer change labeling from males ages 12-17 to ages 16-25, as follows:

On June 25, 2025: Pfizer labeling was finally changed from males ages 12-17 to ages 12-24:
https://labeling.pfizer.com/ShowLabeling.aspx?id=16351
Now the myocarditis risk for boys ages 12-15 was somewhat more consistent with the U.S. Centers for Disease Control and Prevention (CDC)’s “Considerations for COVID-19 vaccination”:
- “People, especially males ages 12–39 years [bold added], should be made aware of the rare risk of myocarditis and pericarditis following receipt of these vaccines…” https://www.cdc.gov/covid/media/pdfs/2025/04/Interim-Clinical-Consideration-for-Use-of-COVID-19-Vaccines.pdf
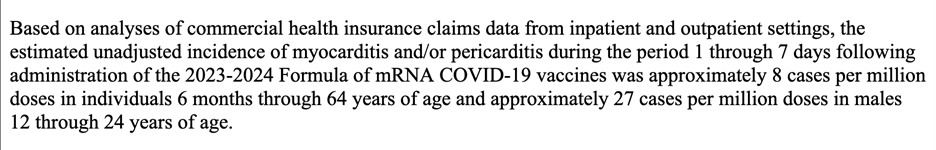
Still, this “estimated unadjusted incidence” specifying male adolescents and young adults could give a false sense of safety for those outside that age range or gender. https://www.thefocalpoints.com/p/covid-19-vaccine-induced-myocarditis-702?r=14jb45&utm_campaign=post&utm_medium=web
Additionally, the new safety information, based on the post-approval study, states: “Initial gadolinium-enhanced cardiac magnetic resonance imaging (CMR) was performed on 216 patients, of whom 177 had late gadolinium enhancement (LGE), a marker of myocardial injury. Among 161 patients who had LGE on initial CMR and who had a follow-up gadolinium-enhanced CMR at a median follow-up of 159 days (interquartile range 78-253 days), 98 had persistence of LGE. Overall, the severity of LGE decreased during follow-up. The clinical and prognostic significance of these CMR findings is not known [bold added].”
Whereas the post-approval study, itself, states: “LGE [late gadolinium enhancement] is associated with a propensity for arrhythmias, heart failure, and sudden cardiac death.” https://www.thelancet.com/action/showPdf?pii=S2589-5370%2824%2900388-2
In all, the safety update is still not considered worthy of a boxed warning by the FDA, “critical for a prescriber to consider.” https://www.fda.gov/media/71866/download
The CDC, as well, focused on mRNA covid vaccine safety at the recent Advisory Committee on Immunization Practices (ACIP) meeting on June 25, 2025. https://www.cdc.gov/acip/downloads/slides-2025-06-25-26/04-Meyer-COVID-508.pdf
Interestingly, the CDC acknowledges increased risk of myocarditis in ages 12+, contrary to the FDA’s initial request to change warnings to 16+:
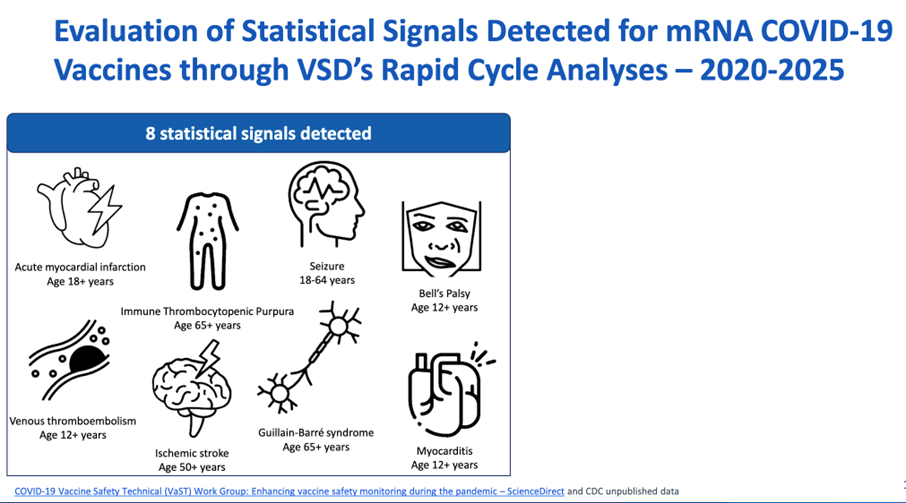
The CDC safety reported that the Vaccine Safety Datalink (VSD) “demonstrated no statistical signals for myocarditis in children [<12 years].”

I hope the raw data from the VSD is available for the ACIP members to independently evaluate, beyond the CDC’s interpretation. According to attorney Aaron Siri, the CDC has dismantled certain VSD data such as that needed for a vaccinated vs. unvaccinated study assessing health outcomes in children: “…you can’t get the VSD data anymore, because what our the CDC has done is it essentially took all that data and returned it to each of the HMOs [health maintenance organizations] to make it unavailable…” https://thehighwire.com/ark-videos/ican-fights-back-support-for-the-injured-data-for-the-people/
In my assessment, criteria for boxed warnings were previously met, but the argument has only strengthened. As the covid vaccines are no longer recommended for healthy children, “[t]here is an adverse reaction so serious in proportion to the potential benefit from the drug (e.g., a fatal, life-threatening or permanently disabling adverse reaction) that it is essential that it be considered in assessing the risks and benefits of using the drug.”
In my opinion, the information in the “New Safety Information” is insufficient to allow a person, or parent, to decide about accepting risk.
As of May 30, 2025, searching the Vaccine Adverse Event Reporting System (VAERS) for preferred term (PT): myocarditis, Pfizer, Moderna and Novavax monovalent only, and excluding unknown manufacturers, there were 432 events of death:
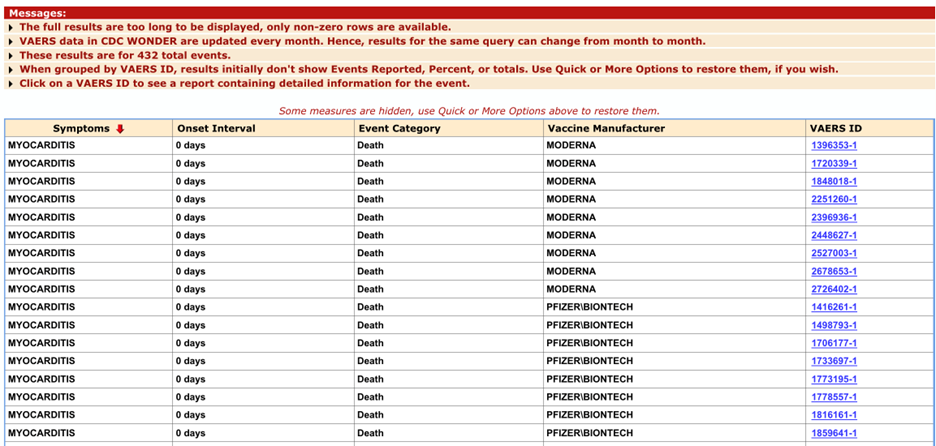
https://wonder.cdc.gov/controller/datarequest/D8;jsessionid=FA2A092F8338C8A0533032BE1829
The United Kingdom is warning its people about fatal myocarditis and pericarditis:
https://www.medicines.org.uk/emc/product/15834/smpc/print
Why can’t we?
The post New Myocarditis Warnings for Covid “Vaccines”: Where is the Black Box? appeared first on DailyClout.
Click this link for the original source of this article.
Author: Sean Probber
This content is courtesy of, and owned and copyrighted by, https://dailyclout.io and its author. This content is made available by use of the public RSS feed offered by the host site and is used for educational purposes only. If you are the author or represent the host site and would like this content removed now and in the future, please contact USSANews.com using the email address in the Contact page found in the website menu.








