The following excerpted case report was published online last month. and yet again shows that inexpensive repurposed compounds like Fenbendazole and Ivermectin may cure cancer better than any legacy Medical-Industrial Complex “treatments.”
Fenbendazole as an Anticancer Agent? A Case Series of Self-Administration
Makis W, Baghli I, Martinez P
Abstract
Background: Fenbendazole (FBZ), an inexpensive and widely accessible anti-parasitic drug used in veterinary medicine, has garnered growing interest for its potential as an anticancer therapy. Preclinical studies suggest that FBZ exerts its anticancer effects through a wide variety of mechanisms. While FBZ has shown promise both in vitro and in vivo studies, clinical evidence supporting its use and efficacy in treating metastatic cancer is currently limited.
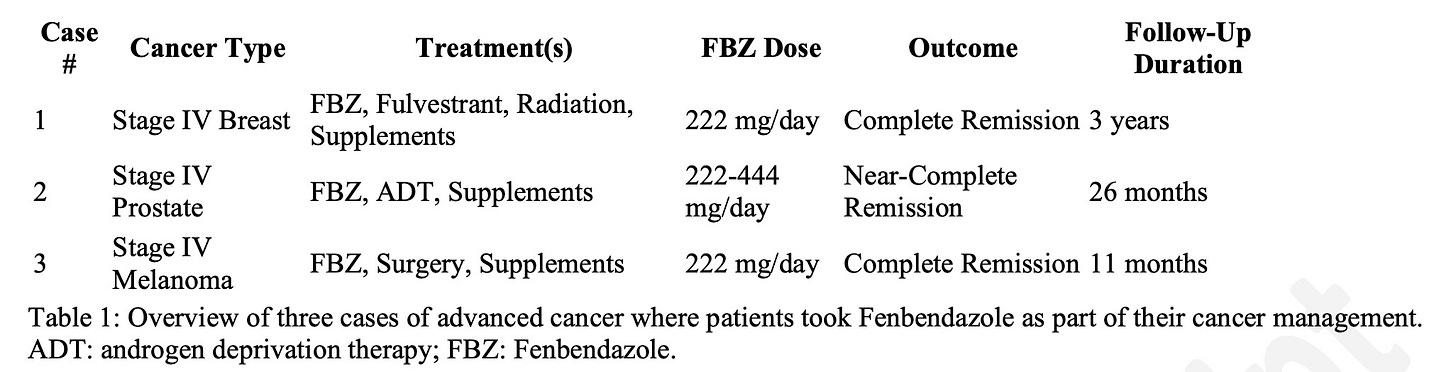
Case Presentations: This report highlights three cases of patients with advanced cancer—including breast, prostate, and melanoma. Two patients achieved complete remission, and one achieved near-complete remission after incorporating FBZ into their treatment regimens alongside other therapies (excluding chemotherapy). All three patients tolerated FBZ without any reported adverse effects, and remission was sustained during follow-up periods ranging from eleven months to nearly three years. Conclusion: FBZ demonstrates potential as a novel promising therapeutic option for repurposing in oncology. Its ability to contribute to tumor regression and achieve disease remission warrants further clinical research to establish its efficacy and optimize its use.
Keywords: Fenbendazole; Veterinary medication; Anti-parasitic; Case report; Repurposed drug, Benzimidazoles; Advanced cancer; Cancer regression.
Background
Fenbendazole (FBZ) is a benzimidazole anthelmintic commonly used to treat a variety of animal parasitic infections. In recent years, the use of FBZ as either a standalone cancer treatment or as a complementary therapy alongside chemotherapy has gained significant attention among individuals battling various types of cancer [1]. FBZ, an inexpensive anti-parasitic drug widely used in veterinary medicine, is readily accessible through animal supply stores, online platforms and pharmaceutical chemical manufacturers. FBZ was originally patented by Hoechst AG (now part of Sanofi) but the patent expired in the early 1990s, making FBZ available as a generic drug. FBZ has shown potential in both in vitro and in vivo models of cancer, as evidenced by studies such as those conducted by Song et al. [2]. Benzimidazoles, including FBZ, exert anticancer effects through several mechanisms: they disrupt microtubule polymerization, induce apoptosis, arrest the cell cycle at the G2/M phase, inhibit angiogenesis, and interfere with both glucose [3] and probably also glutamine [4] metabolic pathways. Although there is increasing interest in FBZ and its potential application for treatment of advanced cancer, the evidence available in the published literature regarding its efficacy remains limited, and there is a substantial lack of clinical research to support its role as an anticancer treatment. This report follows the CARE Checklist and presents three cases of advanced cancer, in which two patients achieved complete remission and one achieved near-complete remission following the use of FBZ.
Case presentations
Case 1
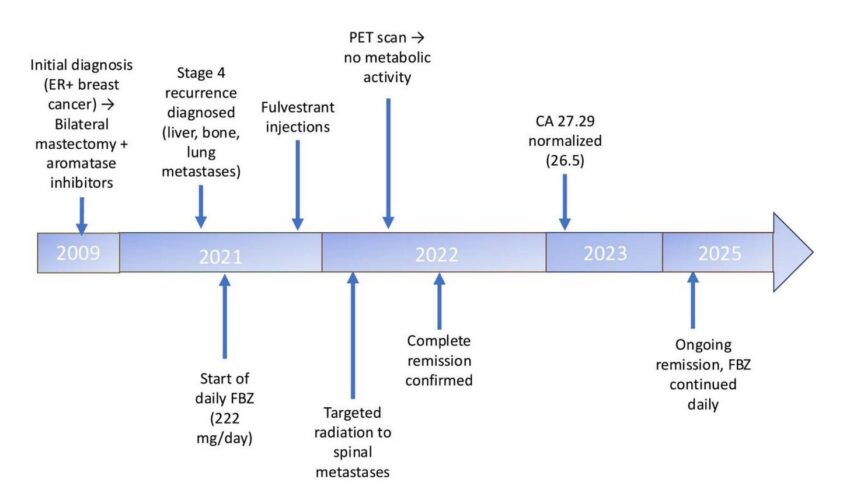
An 83-year-old female was diagnosed with stage 4 breast cancer in October 2021. She was initially diagnosed in 2009 with estrogen receptor-positive breast cancer, treated with bilateral mastectomy, reconstruction, and aromatase inhibitors (later discontinued). She remained disease-free until a recurrence was diagnosed in 2021. Immunohistochemistry revealed strongly positive for CK7 (Cytokeratin 7), Cytokeratin Oscar, GATA3 (GATA Binding Protein 3), and CD68 (Cluster of Differentiation 68). The patient underwent an Esophagogastroduodenoscopy with Endoscopic Retrograde Cholangiopancreatography (ERCP) due to biliary obstruction requiring stent placement. During that procedure, she underwent a Fine Needle Aspiration (FNA) of her liver, which confirmed metastatic breast carcinoma characterized as ER/PR positive and HER-2/neu negative. Ascitic fluid analysis also confirmed metastatic breast carcinoma. Magnetic Resonance Imaging (MRI) of the spine in October 2021 revealed metastatic breast cancer involving multiple bones, including T10, T12, L1, L2, L3, L4, L5, S1, S2, and the iliac bones. A PET/CT scan on December 29, 2021, showed six hypermetabolic lung lesions, the largest located in the central right upper lobe measuring 2.8 × 1.5 cm (SUV max 8.4), a posteromedial right lower lobe lesion measuring 1.8 × 1.4 cm (SUV max 4.6), and a left upper lobe lesion measuring 0.8 cm (SUV max 4.4). There were hypermetabolic liver lesions in the left hepatic lobe, with the index lesion in the medial left hepatic lobe measuring 2.9 × 1.7 cm (SUV max 5.6) and hypermetabolic bone lesions, notably a 5.0 × 2.9 cm lytic lesion in L4 (SUV max 6.8) extending into the spinal canal, and a 2.0 cm lesion in T12 (SUV max 3.5). The patient declined further conventional chemotherapy or radiation therapy and was placed under hospice care. On November 22, 2021, she began self-administering FBZ daily at a dose of 222 mg. In December 2021, she received fulvestrant injections (an estrogen receptor blocker) intended to inhibit cancer growth in a manner similar to restricting glucose. In January 2022, she underwent targeted radiation for two painful spinal metastases. These tumors disappeared rapidly, relieving her pain within a few days. She continued taking 222 mg/day of FBZ for eight months. During this time, her liver enzymes normalized, and her CA 27.29 dropped from 316 (November 2021) to 36.6 (July 2022) (see supplementary material). On April 20, 2022, a PET scan confirmed the absence of any abnormal metabolic activity indicative of cancer. This was corroborated by the steady decline in her CA 27.29 levels, which can lag cancer elimination by several months. In June 2022, the patient was confirmed to have no evidence of active disease. All treatments were discontinued, and she was considered to be in complete remission. Follow-up monitoring was scheduled every 3 to 6 months. Throughout her FBZ treatment, she continued her regular supplementation of vitamin D (5000 IU) and a multivitamin. In July 2022, blood tests revealed elevated Alanine Aminotransferase (ALT) and Aspartate Aminotransferase (AST) levels, suggesting potential liver dysfunction, though it remains unclear whether this was caused by fulvestrant, FBZ, or an interaction between the two. Liver function normalized within weeks, while CA 27.29 levels continued to decline to 37 (July 2022) and 26.5 (February 2023), both values within the normal range. Subsequent PET scans showed no abnormal metabolic activity. The FBZ treatment period revealed no adverse effects at this dosage. The patient remains recurrence-free and continues to take FBZ daily nearly three years after being declared to be in remission. A summary timeline is provided in Figure 1 below.
Case 2
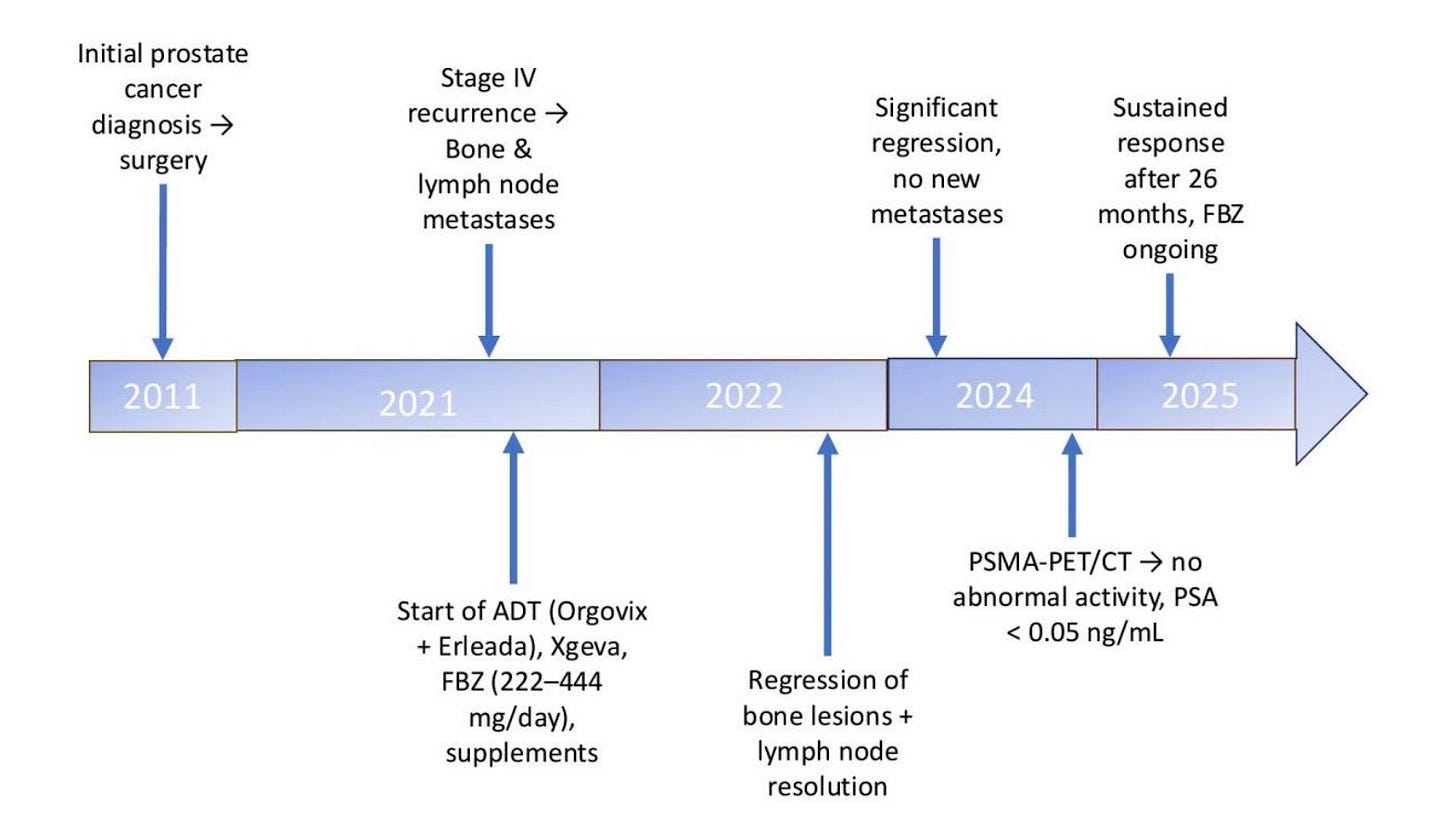
A 75-year-old man was diagnosed in December 2021 with recurrent stage IV prostate cancer and extensive bone metastases. Initially diagnosed ten years earlier and treated surgically, he had undetectable PSA levels for 18 months before a gradual rise indicated recurrence of prostate cancer.. The diagnosis of metastatic cancer was confirmed through imaging studies and elevated PSA levels. Bone scans and CT scans revealed metastases in the spine, pelvic bones, and right humeral head, along with significant lymph node involvement. On a CT abdomen/pelvis with and without IV contrast performed on December 16, 2021, prominent left periaortic lymph nodes were identified, with a representative lymph node measuring 0.8 cm, which was not seen in previous studies. In December 2021, the patient initiated androgen deprivation therapy (ADT) with Orgovix and Erleada, complemented by Xgeva to support bone health. He also added repurposed medications and supplements: vitamin D (5,000–10,000 IU/day) with K2 and magnesium, melatonin (10–40 mg/day), berberine, curcumin, artemisinin, cimetidine, and other compounds with potential anticancer effects. He started taking FBZ in December 2021 (dose range: 222–444 mg/day), usually daily, with occasional dose reductions. In December 2022, after one year of follow-up, regression of bone lesions was observed, and lymph node involvement had fully resolved. In January 2024, after two years of follow-up, imaging confirmed significant regression of bone lesions with no new metastatic sites. The use of FBZ coincided with continued regression of metastatic lesions and sustained undetectable PSA levels. No increase in liver enzymes or any other side effects attributable to FBZ were reported. In April 2024, a PSMA-PET/CT whole body scan revealed that the vast majority of the sclerotic bone lesions demonstrated no abnormal radiopharmaceutical accumulation. A large left renal cortical cyst deforming the kidney had a SUV of 0.5, and no abnormal radiopharmaceutical accumulation was observed within lymph nodes. PSA levels remained undetectable for over two years (<0.05 ng/mL). After 26 months of sustained regression and no new progression, the patient remains in near complete response and continues FBZ with conventional therapy (ADT with Xgeva) . A summary timeline is provided in Figure 2 below.
Case 3
In July 2020, a 63-year-old man presented with a hip growth diagnosed as BRAFV600-mutated stage IIIC melanoma. He began 8 months of adjuvant dabrafenib and trametinib, stopped early (May 2021) due to decreased ejection fraction (EF). After one year of treatment, the patient achieved remission, which lasted until 2023.
On December 12, 2023, however, a biopsy confirmed recurrence: a 1.6 mm ulcerated malignant melanoma located in the lower left abdomen (SOX-10 and pan-melanoma positive). PET-CT showed multiple hypermetabolic foci—peritoneal and retroperitoneal nodules, focal uptake in the stomach and small bowel, lesions in the right gluteus medius, quadratus femoris, and L5 vertebra. Incidental finding included mass-like distal ureter thickening which was confirmed on biopsy to be a different malignancy (urothelial carcinoma). Tempus xF (circulating tumor DNA) showed presence of BRAFV600 mutation, suggestive of the presence of recurrent melanoma. The patient’s oncologist recommended delaying immunotherapy with nivolumab (Opdivo) after biopsy of the recurrent melanoma. During this treatment-free window, he began self-administering FBZ daily (dose range: 222mg – 444mg) in mid-December 2023. The ureteral tumors disrupted urination, necessitating surgery in mid-December 2023. Blood tumor markers, measured as circulating tumor DNA, provided clear evidence of the melanoma progression and subsequent remission. On November 29, 2023, before initiating FBZ, the tumor marker with Signatera test was 123.37. By January 17, 2024, less than seven weeks after starting the treatment, it dropped to 0.38 and reached 0 (zero) by February 21, 2024. During this period, the patient received two doses of nivolumab. Remarkably, at the February 2024 follow-up, imaging and blood tests indicated “no evidence of disease” (NED). Supplements—including ascorbic acid (2,000 mg BID), cephalexin (500 mg single dose), cholecalciferol, CoQ10, cyanocobalamin, and glutathione—were taken throughout treatment and were part of the patient’s regular routine before and after remission. The patient remains melanoma recurrence-free over eleven months after being declared to be in remission. A summary timeline is provided in Figure 3 below.
Conclusion
Despite the limited data and the relatively few studies on FBZ’s anti-oncogenic properties in humans, this case series underscores the importance of further investigation into its potential application as a cancer treatment. The mechanisms by which benzimidazoles, including FBZ, work against cancer cells are well documented in preclinical models, but clinical evidence remains sparse. Given the promising results observed in these cases and the generally favorable safety profile of FBZ, future studies are warranted to assess its efficacy in larger cohorts and explore its potential for repurposing in the treatment of various malignancies. With its low cost and accessibility, FBZ represents a potentially valuable avenue for further exploration, either as a standalone treatment or in combination with traditional therapies, for patients with advanced cancers.
The full research paper:
The very best synergistic ‘holy grail’ cancer cure in plain sight that is also an Alzheimer’s Disease and diabetes treatment may be the following:
New & Improved Synergistic Joe Tippens Protocol
-
Tocotrienol and Tocopherol forms (all 8) of Vitamin E (400-800mg per day, 7 days a week). A product called Gamma E by Life Extension or Perfect E are both great.
-
Bio-Available Curcumin (600mg per day, 2 pills per day 7 days a week). A product called Theracurmin HP by Integrative Therapeutics is bioavailable.
-
Vitamin D (62.5 mcg [2500 IU] seven days a week).
-
CBD oil (1-2 droppers full [equal to 167 to 334 mg per day] under the tongue, 7 days a week) CBD-X: The most potent full spectrum organic CBD oil, with 5,000 milligrams of activated cannabinoids and hemp compounds CBD, CBN & CBG per serving.
-
Fenbendazole (300mg, 6 days a week) or in the case of severe turbo cancers up to 1 gram
-
Ivermectin (24mg, 7 days a week) or in the case of severe turbo cancers up to 1mg/kg/day
-
VIR-X immune support (2 capsules per day)
-
Removing sugars and carbohydrates (cancer food) from your diet and replacing table sugar with a zero glycemic index, zero calorie, keto friendly rare sugar like FLAV-X
Do NOT comply.
Please take advantage of the extended BIGGEST FLASH SALE by using code REAL30 for 30% off on ALL of the products that you have been buying for many years like the Nobel Prize winning miracle compound Ivermectin, the no less miraculous Fenbendazole, Doxycycline, the full spectrum organic CBD oil containing 5,000 milligrams of activated cannabinoids and hemp compounds CBD, CBN & CBG, the powerful immune support nutraceutical and spike support formula VIR-X, and the sugar craving reducing, blood sugar balancing and even anti-cancer allulose sugar substitute FLAV-X!
Please note that all of the amazing products that this Substack has been promoting for many years now are exclusively offered by RESOLVX HEALTH.
This BIGGEST FLASH SALE has been extended to Sunday, June 29th (midnight eastern time), 2025.
Upon adding products to your cart, please go to the cart icon at the top right corner of your browser page and click it, then choose the VIEW CART option whereby you will be redirected to a page where you can enter the code REAL30 in the Use Coupon Code field.
Please contact the company directly with any product questions: [email protected]
(Please note that any other company offering RESOLVX HEALTH products is selling you counterfeit items.)
Click this link for the original source of this article.
Author: 2nd Smartest Guy in the World
This content is courtesy of, and owned and copyrighted by, https://2ndsmartestguyintheworld.substack.com and its author. This content is made available by use of the public RSS feed offered by the host site and is used for educational purposes only. If you are the author or represent the host site and would like this content removed now and in the future, please contact USSANews.com using the email address in the Contact page found in the website menu.