by DR. WILLIAM MAKIS MD
Last year I published one of the most popular articles on Fenbendazole and Cancer Treatment ever published, which went viral internationally (even Joe Rogan read my article on his show):
(Oct.3, 2023) – FENBENDAZOLE and CANCER – at least 12 Anti-Cancer mechanisms of action. Not approved by FDA. Cheap. Safe. Kills aggressive cancers. Why no Clinical Trials? Nine research papers reviewed.
10 NEW STUDIES SINCE LAST ARTICLE!
-
(2024 Apr, Rodrigues et al) – Repurposing mebendazole against triple-negative breast cancer CNS metastasis
-
(2024 Feb, Eid et al) – Investigating the Promising Anticancer Activity of Cetuximab and Fenbendazole Combination as Dual CBS and VEGFR-2 Inhibitors and Endowed with Apoptotic Potential
-
(2024 Feb, Park et al) – The microtubule cytoskeleton: A validated target for the development of 2-Aryl-1H-benzo[d]imidazole derivatives as potential anticancer agents
-
(2024 Jan, Matsuo et al) – Parbendazole as a promising drug for inducing differentiation of acute myeloid leukemia cells with various subtypes
-
(2023, Dec, Iragavarapu-Charyulu et al) – A novel treatment to enhance survival for end stage triple negative breast cancer using repurposed veterinary anthelmintics combined with gut‑supporting/immune enhancing molecules
-
(2023 Nov, Aliabadi et al) – In vitro and in vivo anticancer activity of mebendazole in colon cancer: a promising drug repositioning
-
(2023 Nov, Jung et al) – Fenbendazole Exhibits Differential Anticancer Effects In Vitro and In Vivo in Models of Mouse Lymphoma
-
(2023 Sep, Garg et al) – Network pharmacology and molecular docking study-based approach to explore mechanism of benzimidazole-based anthelmintics for the treatment of lung cancer
-
(2023 Jun, Mukherjee et al) – Ketogenic diet as a metabolic vehicle for enhancing the therapeutic efficacy of mebendazole and devimistat in preclinical pediatric glioma
-
(2023 Feb, Lee et al) – Benzimidazole and its derivatives as cancer therapeutics: The potential role from traditional to precision medicine
MY TAKE ON MOST RECENT RESEARCH:
There is still some research being done on Fenbendazole & Cancer, but researchers are focusing more on other related compounds in the “Benzimidazole family”, namely Mebendazole, but also Albendazole, Parbendazole. Why?
COST:
Fenbendazole is cheap. If big pharma is going to make money (especially in cancer treatment), they need an expensive compound and Fenbendazole isn’t it.
Fenbendazole is not FDA approved. It’s dirt cheap.
Mebendazole is FDA approved. It’s expensive.
Albendazole is FDA approved. It’s very expensive.
Dr.Thomas Seyfried talks about the cost of Mebendazole
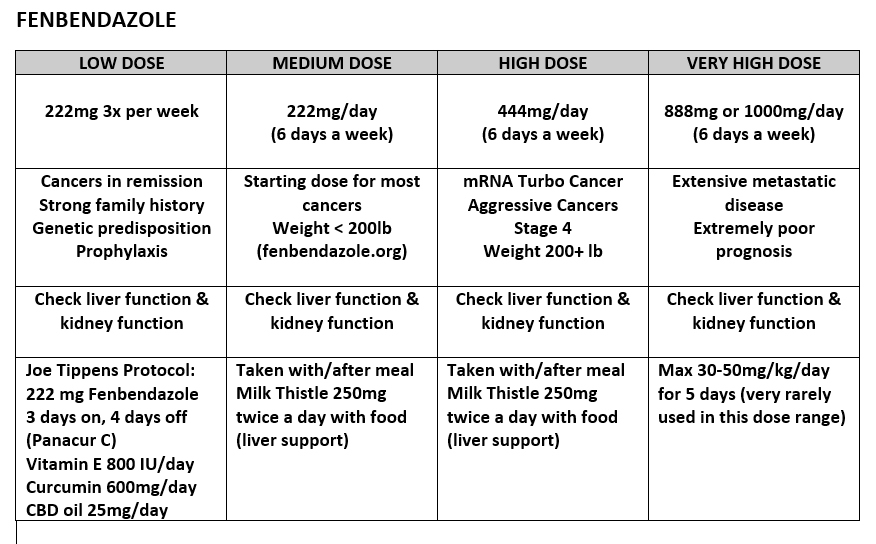
PRACTICAL APPROACH TO USING FENBENDAZOLE IN CANCER TREATMENT (Disclaimer: the following is not medical advice)
In “Fenbendazole and Cancer Part 1”, I covered all the mechanisms of action that Fenbendazole has shown against cancer in many in vitro and in vivo studies.
The 10 new studies published in 2023-2024 only confirm what we already know from previous studies. Fenbendazole, Mebendazole, Albendazole are highly effective against many cancers.
There is one article I want to highlight though because it has an extremely important concept:
-
(2023 Jun, Mukherjee et al) – Ketogenic diet as a metabolic vehicle for enhancing the therapeutic efficacy of mebendazole and devimistat in preclinical pediatric glioma
-
“This study investigated the influence of nutritional ketosis on the therapeutic action of mebendazole (MBZ) and devimistat (CPI-613) against the highly invasive VM-M3 glioblastoma cells in juvenile syngeneic p20-p25 mice”
-
“maximum therapeutic benefit of mebendazole and CPI-613 on tumour invasion and mouse survival occurred only when the drugs were administered together with a ketogenic diet (KD)
KETOGENIC DIET IS CRUCIAL IN CANCER!!!
Since my previous Fenbendazole article, I‘ve had 1000s of questions sent to me. Not about mechanisms of action against Cancer. But about practical use – how to use Fenbendazole or Mebendazole to treat Stage 4 Cancers, what formulations, what doses?
The goal of this article (Part 2) is to answer many of those questions to the best of my ability.
EXPERIMENTAL CANCER PROTOCOLS:
I propose the following thought experiment & hypothetical “Experimental Protocols” for Turbo Cancer Treatment:
-
You can look at fenbendazole.org for suggested dosing and dose calculator
-
Dr.Tom Rogers, Founder of “Performance Medicine” has similar protocols.
-
For anyone COVID-19 mRNA Vaccinated diagnosed with cancer (Turbo Cancer), I’d probably be looking at starting at 444 mg a day.
-
For particularly aggressive Turbo Cancers or bad cases, I’d even consider pushing towards 888 mg/day (444 mg twice a day) or 1000 mg/day.
-
Highest dosing I’ve seen is 30-50mg/kg/day for 5 days, based on the “Merck Manual”, however very few claim to have taken this dose.
-
Fenbendazole can elevate liver function tests, so it would be a good idea to have a family doctor monitor those
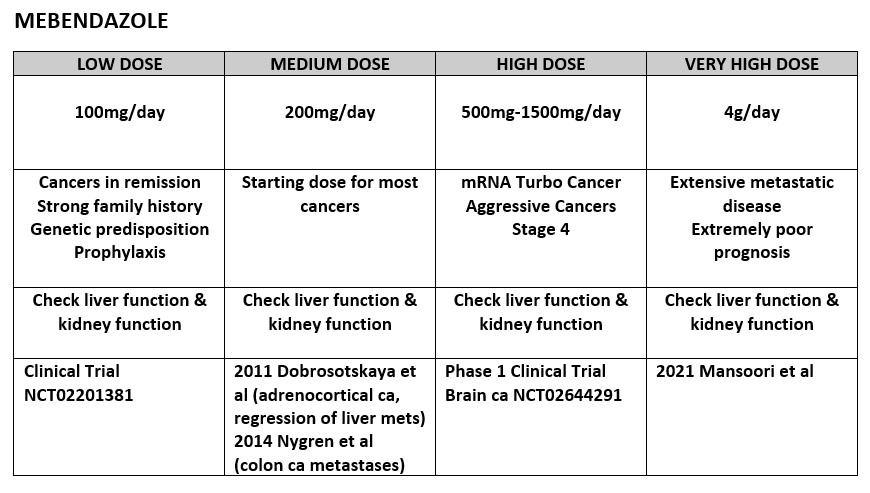
WHAT ABOUT MEBENDAZOLE?
-
Here are the references for this dosing schedule:
-
For Maximum dose of 4g/day being safe, that’s from a Phase 2 Clinical Trial for Gastrointestinal Cancer: (2021 Mansoori et al)
-
(2021 Chai et al) – summarizes the various studies that have looked at Mebendazole in Cancer and the doses used.
-
500mg-1500mg/day (Phase 1 Clinical Trial, pediatric brain tumors)
-
200mg/day (2011 Dobrosotskaya et al) (adrenocortical ca)
-
200mg/day (2014 Nygren et al) (colon ca lung and LN mets)
-
100mg/day (Clinical Trial, UK)
-
-
So far, several studies in the literature have used 200mg/day with some success, however given that it is safe to go up to 4g/day, when we’re dealing with aggressive mRNA Induced Turbo Cancers, 200mg/day is probably not sufficient.
-
Why Mebendazole over Albendazole (2021 Chai et al):
-
“However, because of the toxicity of albendazole, for example, neutropenia due to myelosuppression, if high doses are used for a prolonged time, mebendazole is currently more popularly used than albendazole in anti-cancer clinical trials.”
-
My Take…
My goal with these articles, is to provide as much clear information as possible for someone dealing with cancer or mRNA Induced Turbo Cancer.
This article deals with the practical approach to using Febendazole or Mebendazole.
Hypothetical – if I was diagnosed with mRNA Induced Turbo Cancer, as a 40s year old male, I’d be strongly looking at taking a combination of Ivermectin (1mg/kg/day) and Fenbendazole (444mg/day). This decision would be made based on dozens of peer-reviewed papers that have been published, previous and ongoing Clinical Trials, etc.
Everyone’s situation is different, however, it is important to arm yourself with medical knowledge that cancer doctors (Oncologists) will simply not give you, because they either don’t know it, or they won’t risk their careers to save you.
2nd Smartest Guy’s Take…
The holy grail turbo cancer cure may just be the synergistic combination therapy of Fenbendazole AND Ivermectin as follows:
New & Improved Joe Tippens Protocol
-
Tocotrienol and Tocopherol forms (all 8) of Vitamin E (400-800mg per day, 7 days a week). A product called Gamma E by Life Extension or Perfect E are both great.
-
Bio-Available Curcumin (600mg per day, 2 pills per day 7 days a week). A product called Theracurmin HP by Integrative Therapeutics is bioavailable.
-
CBD oil (1-2 droppers full [equal to 25mg per day] under the tongue, 7 days a week) https://www.soothingsolutionscbd.com/product/3500mg-full-spectrum-cbd-tincture/ (Please use code 2SGPET for 10% off on this full spectrum CBD oil.)
-
Fenbendazole (300mg, 7 days a week) or in the case of severe turbo cancers up to 450mg
-
Ivermectin (24mg, 7 days a week) or in the case of severe turbo cancers up to 1mg/kg/day
-
Removing sugars and carbohydrates from one’s diet is crucial during this protocol.
Please use code SYN20 for 20% off on Ivermectin, Fenbendazole, and Doxycycline.
Upon adding products to your cart, please go to the cart icon at the top right corner of your browser page and click it, then choose the VIEW CART option whereby you will be redirected to a page where you can enter the code SYN20 in the Use Coupon Code field.
Sale ends Monday, April 15th, 2024.
Please contact the company directly with any product questions: [email protected]
They want you dead.
Do NOT comply.
Click this link for the original source of this article.
Author: 2nd Smartest Guy in the World
This content is courtesy of, and owned and copyrighted by, https://2ndsmartestguyintheworld.substack.com and its author. This content is made available by use of the public RSS feed offered by the host site and is used for educational purposes only. If you are the author or represent the host site and would like this content removed now and in the future, please contact USSANews.com using the email address in the Contact page found in the website menu.